Print Friendly
Transcranial Magnetic Stimulation for Depression: One-Year Durability of Response and Remission
Transcranial Magnetic Stimulation for Depression:
One-Year Durability of Response and Remission
David L. Dunner, MD, FACPsych
Director, Center for Anxiety and Depression, Mercer Island, WA; Professor Emeritus, Department of Psychiatry and Behavioral Science, University of Washington
First published in Psychiatry Weekly, Volume 9, Issue 12, December 15, 2014
Introduction
Transcranial magnetic stimulation (TMS) therapy for treatment refractory depression (TRD) has grown slowly in the United States since the FDA cleared TMS devices for marketing in 2008. Dr. David Dunner, a TMS practitioner who specializes in treating patients with TRD, says that nearly 500 TMS devices have been sold in the US since that time. In 2014, following the release of more adequately powered efficacy studies, the FDA granted approval for the use of TMS in treating depression in episodes where multiple previous trials of pharmacotherapy had failed.
TMS is not widely available in the US, which Dr. Dunner attributes to several factors. First, insurance reimbursement for TMS is generally spotty, although it continues to improve slowly. “I think it’s been one of the major factors deterring more patients with severe TRD from receiving TMS,” he says.
Secondly, many psychiatrists are in solo private practice, and the extra space and additional clinical staff required to run a TMS unit may well be out of reach. For now, TMS units are generally found in selected private offices, academic centers, and large institutions.
TMS Research
In 2012, Carpenter and colleagues published a naturalistic observational study of the effects of acute TMS treatment.1 In that study, 307 depressed outpatients received TMS treatment at 42 clinical sites. Patients were mostly female (66.8%) with a mean age of 48.6 years. A majority (92.5%) had a history of recurrent major depression, 43.6% had a history of being hospitalized for depression, and 5.2% had a history of ECT treatment. The mean number of antidepressant treatments in the current episode was 3.6, and the mean number of “adequate” treatments (taking into account dose and duration) was 2.5. The acute TMS treatment phase comprised an average number of 28.3 TMS sessions. Most patients continued their current pharmacotherapy regimen during TMS treatment.
The main outcome measure in the Carpenter study was change in the Clinical Global Impressions (CGI) Severity of Illness scores. The study results did show a significant reduction in CGI scores at the conclusion of acute treatment (–1.9 ± 1.4, P < .0001), with a response rate of 58% and a remission rate of 37.1%. Similar results were found using the 9-item Patient Health Questionnaire (PHQ-9) and the self-report version of the Inventory of Depressive Symptomatology (IDS-SR).
Especially against the background of a 15% chance of improvement, the fact that this group displayed response rates well over 50% is impressive.
Durability of Response to TMS
“After failure of 3 or more different treatments for depression, the chance of getting better with the following treatment is about 15%, as reported by the STAR*D trial and other studies,” says Dr. Dunner. “This was a seriously ill sample, wherein 43% of participants had been hospitalized for depression, and 15% had a comorbid anxiety disorder. Especially against the background of a 15% chance of improvement, the fact that this group displayed response rates well over 50% is impressive.”
Using the same sample population as in the Carpenter study, Dunner and colleagues conducted a follow-up study to evaluate the long-term durability of TMS treatment effects following the acute treatment phase.2 The 52-week follow-up involved 257 patients who agreed to be followed and included assessments at 3, 6, 9, and 12 months, using several clinical measures to track symptoms.
Click to Enlarge
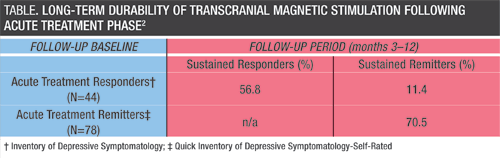
“We found that whatever the status of the patient’s treatment response following the acute phase, that status was largely maintained during follow-up,” says Dr. Dunner. “Approximately 30% of patients who remitted after acute TMS treatment relapsed during the follow-up period; relapse generally occurred during the first 6 months of follow-up, but most patients who worsened went from remission to response.”
“The immediate efficacy of acute TMS treatment in this sample was quite good,” continues Dr. Dunner. “Showing such a high level of initial symptom improvement, and then for such a large proportion to maintain that improvement over 1-year follow-up, are both impressive. This is a very meaningful response rate for a population with a history of TRD and multiple treatment failures.”
This interview was conducted on November 3, 2014 by Lonnie Stoltzfoos
Disclosure: Dr. Dunner has received grant support/funding from Cyberonics and Neuronetics; has served on the advisory board of Bristol-Myers Squibb, Jazz Pharma, and MedDEV Inc. Dr. Dunner owns a Neuronetics TMS device for use in clinical practice.
References:
1. Carpenter LL, Janicak PG, Aaronson ST, et al. Transcranial magnetic stimulation (TMS) for major depression: a multisite, naturalistic, observational study of acute treatment outcomes in clinical practice. Depress Anxiety. 2012;29:587-596.
2. Dunner DL, Aaronson ST, Sackeim HA, et al. A multisite, naturalistic, observational study of transcranial magnetic stimulation for patients with pharmacoresistant major depressive disorder: durability of benefit over a 1-year follow-up period. J Clin Psychiatry. 2014 Sep 16. [Epub ahead of print]