Fatigue in Cancer Patients and Survivors: Mechanisms and
Treatment
Julienne E. Bower, PhD
Dr. Bower is assistant professor in the Department of
Psychiatry and Biobehavioral Sciences at David Geffen School of Medicine at the
University of California, Los Angeles, and research scientist in the Cousins Center for Psychoneuroimmunology at the UCLA Neuropsychiatric Institute.
Disclosure: Dr. Bower reports no
affiliation with or financial interest in any commercial organization that
might pose a conflict of interest.
Funding/support:
This work was supported by a Career Development Award from the National Cancer
Institute (grant no. K07 CA09047) awarded to Dr. Bower.
Please direct all correspondence to: Julienne E.
Bower, PhD, Cousins Center for Psychoneuroimmunology, 300 UCLA Medical Plaza,
Rm 3306, Los Angeles, CA 90095-7076; Tel: 310-794-9383; Fax: 310-794-9247;
E-mail: [email protected].
Focus Points
•
Fatigue is the most common and distressing symptom experienced by cancer
patients.
• Preliminary
evidence suggests that persistent fatigue in breast cancer survivors is
associated with alterations in markers of pro-inflammatory cytokine activity
and in T-lymphocytes, which is suggestive of a chronic inflammatory process.
•
Fatigue and depression are strongly correlated in cancer patients; however,
fatigue cannot be explained entirely by mood disturbance and may be mediated by
a different biological mechanism.
• Randomized
clinical trials have demonstrated that both exercise and psychoeducational
interventions are effective in reducing cancer-related fatigue.
Abstract
Fatigue is one of the most common and distressing side
effects of cancer and its treatment, occurring in approximately 60% to 96% of
cancer patients. Cancer-related fatigue is more pervasive, debilitating, and
longer lasting than normal fatigue and is not relieved by adequate sleep or
rest. The mechanisms underlying fatigue in cancer patients and survivors are
not known, although correlates of fatigue have been identified. Emerging
evidence suggests that pro-inflammatory cytokines are involved in
cancer-related fatigue. Psychological factors are also strongly correlated with
fatigue, particularly depressed mood. However, cancer-related fatigue cannot be
explained entirely by depression. Exercise and psychoeducational interventions
are effective in reducing fatigue and improving quality of life during and
after cancer treatment.
Introduction
With advances in detection and treatment, survival times for
many cancers have increased significantly in recent years. In 1971, there were
approximately 3 million cancer survivors in the United States; by 2001, this
number had increased to 9.8 million.1 As survival times increase,
addressing the impact of cancer and its treatment on long-term outcomes has
become increasingly important. In particular, better understanding and
management of cancer-related symptoms is critical for reducing suffering and
improving quality of life in cancer survivors.
Fatigue is one of the most common and distressing side
effects of cancer and its treatment. An emerging literature has examined the
prevalence and correlates of cancer-related fatigue and the efficacy of
treatments designed to reduce fatigue in cancer patients and survivors. This
article will review the literature on cancer-related fatigue, focusing on
potential psychological and biological mechanisms and the association between
fatigue and depression. In addition, research on treatments for fatigue will be
considered.
Prevalence of Fatigue
Studies have shown that approximately 60% to 96% of cancer
patients experience fatigue at some point during the course of cancer
treatment.2 Fatigue has been documented across a range of different
types of cancer and cancer treatments, including radiation therapy,
chemotherapy, bone marrow transplant, and biological response modifiers. The
intensity and duration of fatigue experienced by cancer patients during
treatment is significantly greater than that experienced by healthy controls.3
Perhaps more important, fatigue causes significantly more disruption in the
lives of cancer patients than healthy controls.4 In a nationwide
survey of cancer patients, over 50% reported that fatigue affected their
ability to work, their physical and emotional well-being, their social
activity, and their ability to enjoy life in the moment.5
Although fatigue typically declines after completion of
cancer treatment, there is growing evidence to suggest that fatigue may persist
for months or even years in a substantial minority of patients. Bower and
colleagues6 found that approximately 30% of breast cancer survivors
reported significant fatigue an average of 3 years after cancer diagnosis. A
similar high incidence of fatigue (26%) was found in a large cohort of
Hodgkin’s disease survivors assessed at an average of 12 years after treatment.7
Consistent with research conducted with cancer patients during treatment,
fatigue has a detrimental impact on all aspects of quality of life in cancer
survivors.6,8
Definition and Mechanisms
Fatigue is a nonspecific, multidimensional construct that is
generally thought to involve subjective feelings of tiredness, weakness, and/or
lack of energy. Cancer-related fatigue differs from “normal” fatigue due to
lack of sleep or overexertion in several ways. First, fatigue in cancer patients
is more pervasive, more debilitating, and longer-lasting. Second, it involves
physical, mental, and emotional fatigue. Third, cancer-related fatigue is not
relieved by adequate sleep or rest. These components are captured in a
description of fatigue9 provided by an oncologist and cancer
survivor:
During cancer therapy, I always felt the exhaustion of prolonged exercise but
without any positive attributes. . . .
My limbs felt heavy. The quality of my sleep was changed. The mere act of
sleeping itself seemed like work sometimes. . . . My brain felt tired and so
did my spirits. I seemed to have lost my zest for life.
In 1998, a
multidisciplinary group of medical practitioners, researchers, and patient
advocates developed diagnostic criteria to define a syndrome of cancer-related
fatigue.10 The key criteria were significant fatigue, diminished
energy, or increased need to rest, disproportionate to any recent change in
activity level that is present every day or nearly every day during the same
2-week period in the last month. Examples of other symptoms include weakness or
heaviness, diminished concentration or attention, decreased motivation or
interest in usual activities, unrefreshing or nonrestorative sleep, and
postexertional malaise. These symptoms must cause clinically significant
distress or impairment, result from cancer or cancer treatment, and cannot be
primarily caused by a comorbid psychiatric disorder.
Despite its prevalence,
the mechanisms that underlie the onset and persistence of cancer-related
fatigue have not been determined. Investigators have proposed that fatigue may
be caused by the disease itself, by treatments for the disease, by physical
symptoms or conditions resulting from the disease or its treatment, by
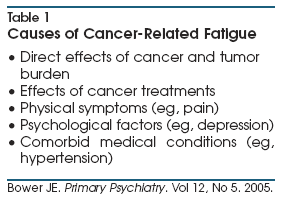
psychological responses to cancer, and by comorbid medical conditions (Table
1).11 Interestingly, disease and treatment-related factors are not
consistently associated with fatigue. For example, research conducted with
breast cancer patients has shown no relationship between fatigue and initial
disease stage, type of surgery, length of chemotherapy treatment, or use of
tamoxifen.8,12,13 There is mixed evidence for an association between
receipt of adjuvant chemotherapy and fatigue in breast cancer patients; several
studies have not found significant differences between patients treated with
surgery, radiation therapy, and/or chemotherapy,6 while others have
documented higher levels of fatigue among former chemotherapy patients compared
to those treated with radiation therapy alone.14
Biological Correlates of
Fatigue
Several studies have examined biological correlates of
fatigue, with mixed results. There is some evidence that low hemoglobin is
associated with increased fatigue15 and, among anemic patients,
increases in hemoglobin following treatment with darbepoetin-a are associated with improvements
in fatigue and quality of life.16 However, the association between
fatigue and hemoglobin is typically modest and has not been documented in all
studies.17 Moreover, low hemoglobin does not explain all of the
variance in fatigue even in the positive reports.15,18 Low serum
albumin has also been correlated with cancer-related fatigue in a study
conducted with patients undergoing treatment for leukemia and non-Hodgkin’s
lymphoma.19
Another potential biological mechanism for cancer-related
fatigue may come from changes in the immune system induced by cancer or cancer
treatment, including release of pro-inflammatory cytokines interleukin (IL)-1β, IL-6, and tumor necrosis
factor (TNF)-α. Basic research20
on neuro-immune signaling in animal models has shown that inflammatory stimuli
can signal the central nervous system to generate fatigue, as well as changes
in sleep, appetite, mood, and social/sexual behavior. Conversely, antagonists
or synthesis blockers of cytokines abolish these effects. In cancer patients,
pharmacologic doses of cytokines (eg, immunotherapy with IL-2) lead to fatigue,
decreased activity, sleep disturbance, depressed mood, and pain.21
Similarly, it appears that acute induction of cytokines in healthy individuals
has behavioral effects, leading to fatigue, depressed mood, anxiety, and
cognitive and sleep disturbance.22,23
Investigators have begun to examine the role of
pro-inflammatory cytokines and the cytokine network in cancer-related fatigue.
In an early study conducted with prostate cancer patients,24 fatigue
and serum levels of IL-1β
both increased during radiation therapy. A positive correlation between
inflammatory markers and fatigue has been documented in more recent research
conducted with breast and lung cancer patients undergoing chemotherapy.25
However, fatigue was not correlated with changes in pro-inflammatory cytokines
in breast cancer patients undergoing radiation therapy.17
It may be difficult to detect an association between fatigue
and pro-inflammatory cytokines during cancer treatment when the immune system
is rapidly responding to tissue damage and other treatment-related changes.
Thus, evaluation of cancer survivors may yield a clearer picture of the
relation between cytokines and fatigue. Bower and colleagues26
compared breast cancer survivors with persistent fatigue to a control group of
nonfatigued survivors. Both groups had completed treatment at least 2.5 years
previously and were disease-free, with no immune-related medical conditions.
Fatigued survivors showed significant elevations in several markers of pro-inflammatory
cytokine activity compared to nonfatigued controls, including IL-1 receptor
antagonist, soluble TNF receptor type II, and neopterin. These molecules can be
measured more reliably in serum than the pro-inflammatory cytokines that induce
their production, and may provide a better measure of cytokine activity.
Elevations in inflammatory markers were coupled with elevations in CD4+
T-lymphocytes, suggesting a chronic inflammatory process involving the T-cell
compartment.27 Fatigued survivors also reported behavioral changes
consistent with pro-inflammatory cytokine activity, including depressed mood,
sleep disturbance, decreased activity, and cognitive disturbance.26
There are several mechanisms through which chronic
inflammation might develop or persist in cancer patients, including alterations
in the hypothalamic-pituitary-adrenal (HPA) axis. Adrenal cortex-derived
steroids have potent effects on pro-inflammatory cytokine production and
activity,28 and disturbances in HPA-axis function have been observed
in other conditions characterized by inflammation and fatigue.29
There may be a dysregulation in HPA axis responsiveness among breast cancer
survivors with persistent fatigue. Fatigued survivors show lower levels of
morning serum cortisol,26 flattened diurnal cortisol slopes,30
and a blunted cortisol response to acute psychosocial stress compared to
nonfatigued controls.31
Fatigue and Depression
Fatigue often co-occurs with physical and psychological
symptoms in cancer patients, including menopausal symptoms, pain, and sleep
disturbance.6,12 Perhaps the strongest and most consistent correlate
of cancer-related fatigue is depression. In a recent review of 30 studies
conducted with cancer patients, the correlation between fatigue and depression
ranged from 0.18–0.80, with an average correlation of 0.54.32
One potential explanation for the high correlation between
symptoms of fatigue and depression is methodological, as instruments assessing
depression typically include symptoms of fatigue. However, removing
fatigue-related items from depression inventories does not appreciably reduce
the association between depression and fatigue.6,32 In addition,
fatigue is also highly correlated with measures of depressed mood that do not
include neurovegetative symptoms.
A second possibility is that fatigue may lead to symptoms of
depression due to its interference with normal activities. The disruption
caused by fatigue may be particularly problematic for cancer survivors, who
have completed cancer treatment and are ready to resume their normal lives.
Indeed, breast cancer survivors reported that fatigue caused greater
interference with mood than a control group of women with no cancer history.4
A third possibility is
that fatigue occurs secondary to mood disturbance in cancer patients. Fatigue
is a prominent symptom of depression, which is elevated in cancer patients
relative to the general population.33 However, several lines of
evidence suggest that cancer-related fatigue cannot be explained entirely by
presence of depression. First, cancer patients with persistent fatigue do not
necessarily report depressed mood. For example, Bower and colleagues6
found that approximately 50% of fatigued breast cancer survivors scored below
the clinical cut-off of 16 on the Center for Epidemiologic Studies Depression
Scale. Second, prospective studies have shown that fatigue and depressive
symptoms follow different trajectories over the course of cancer treatment.
Visser and colleagues34 found that while fatigue either increased or
remained stable after onset of radiation therapy, depression decreased. Nine
months later, fatigue had decreased whereas levels of depression remained
stable. Third, pretreatment depression appears to be a poor predictor of
fatigue severity after treatment. In a study of patients undergoing radiation
therapy, pretreatment depressed mood explained only 4% of the variance in
post-treatment fatigue.34 Further, the presence of a psychiatric
disorder before cancer diagnosis was not associated with fatigue severity after
chemotherapy in a sample of breast cancer survivors, although psychiatric
history is known to be a risk factor for depression after cancer diagnosis.8
Finally, it is possible
that fatigue and depression may be driven by a common underlying biological
disturbance. As described above, pro-inflammatory cytokines have been
associated with both fatigue and depressed mood in cancer patients and healthy
individuals; furthermore, there is evidence that individuals with clinical
depression have elevated serum levels of pro-inflammatory cytokines and markers
of cytokine activity, including cancer patients with depression.35
The research of Bower and colleagues26 with breast cancer survivors
indicates that women with persistent fatigue display both depressed mood and
elevated markers of pro-inflammatory cytokine activity. However, none of these
inflammatory markers were correlated with depressed mood in these patients, nor
did controlling for depression alter the association between fatigue and immune
alterations.26 These results suggest that pro-inflammatory cytokine
activity may be specifically related to energy symptoms rather than depression
in this patient group.
Pro-inflammatory cytokines
are also known to induce changes in monoamines, and may influence both fatigue
and depression through their effects on serotonin pathways.36 To
test this hypothesis, Morrow and colleagues37 conducted a randomized
controlled trial of paroxetine for fatigue in cancer patients undergoing
chemotherapy. Of the 704 eligible patients, 549 (78%) reported fatigue at
baseline and were randomized to receive either 20 mg/day of paroxetine or
placebo during their second cycle of chemotherapy. Patients were reassessed
during their fourth cycle of chemotherapy, approximately 8 weeks later. Results
documented significant reductions in depressive symptoms among patients treated
with paroxetine compared to controls. However, paroxetine had no effect on
fatigue, even among patients who reported more severe fatigue at baseline. It
should be noted that paroxetine is sedating in some and may itself increase
fatigue.
Another trial38
examined the effect of paroxetine among patients with malignant melanoma
undergoing treatment with interferon-α (IFN-α). Depression and fatigue are both common side
effects of IFN-α treatment, as are
anhedonia, impaired concentration, and sleep disturbance.39 Forty
patients with malignant melanoma were randomized to receive either paroxetine
or placebo beginning 2 weeks before initiation of IFN-α and continuing over the first 12 weeks of IFN-α therapy. Paroxetine treatment significantly
reduced the incidence of major depression over the 12-week study period (11%
incidence of depression in the paroxetine group versus 45% incidence in the
placebo group). Examination of symptom clusters indicated that depressive (ie,
depressed mood, guilt, suicidal thoughts, anhedonia) and cognitive symptoms
emerged at week 8 of IFN-α therapy
in the placebo group and were blocked by treatment with paroxetine.40
However, neurovegetative and somatic symptoms, including fatigue, emerged at
week 2 of IFN-α therapy in both groups
and were only partially blocked at week 8 by paroxetine treatment. Thus, it
appeared that depressed mood and fatigue had a different course during IFN-α treatment, and that paroxetine had a differential
effect on these symptoms.
Overall, there is
compelling evidence that fatigue is distinguishable from depression in cancer
patients and may have distinct biological origins. Moreover, these results
suggest that specific cytokine signals may be related to specific symptoms or
symptom clusters, rather than depression in general, in cancer patients.
Fatigue, mood changes, sleep disturbance, pain, cognitive changes, and other
aspects of sickness behavior may be differentially influenced by cytokines and
other biological signals.
Treatment of Cancer-Related Fatigue
A growing number of
controlled intervention studies have specifically targeted fatigue in cancer
patients. Most of this research has focused on exercise and has shown
consistently positive results. A recent review41 found that all of
the published exercise trials demonstrated lower levels of fatigue in cancer
patients who exercised compared to control or comparison groups. Positive
effects were demonstrated across a range of exercise programs, from home-based
walking programs to supervised laboratory regimens, and across a range of
cancer populations. Aerobic exercise was particularly effective, with fatigue
levels approximately 40% to 50% lower in exercising subjects. Although most
trials have been conducted with patients undergoing cancer treatment,
beneficial effects have also been observed in research conducted with cancer
survivors.42
Psychosocial interventions have also shown beneficial effects
on fatigue. For example, an educational group intervention designed to provide
information about cancer and ways to manage the disease had positive effects on
vitality and physical functioning in women undergoing treatment for breast
cancer,43 with the beneficial effects of treatment on vitality
maintained over a 3-year follow-up.44 Similarly, a psychoeducational
group intervention emphasizing patient education and coping skills training led
to improvements in fatigue, vigor, and depressed mood among patients with
malignant melanoma.45 Other forms of group therapy (ie, supportive
expressive group therapy) and individual therapy have also shown beneficial
effects on fatigue.46,47 Effective treatments for fatigue do not
necessarily require in-person interaction; for example, a patient
self-administered form of stress management training demonstrated beneficial
effects on vitality, physical function, and mental health among breast cancer
patients undergoing chemotherapy.48
Pharmacologic treatments for cancer-related fatigue include
erythropoietin (for chemotherapy-induced anemia) and psychostimulants. As noted
above, treatment with erythropoietin leads to increases in hemoglobin and
concurrent improvements in fatigue and physical function among cancer patients
with anemia.16 However, as most cancer patients are not anemic,
erythropoietin is unlikely to be a viable treatment for most cases of
cancer-related fatigue. There is preliminary evidence that psychostimulants may
have beneficial effects on fatigue. In two open-label studies49,50
conducted with advanced cancer patients, methylphenidate led to rapid
improvements in fatigue and other symptoms.
Overall, these results provide strong evidence that exercise
interventions lead to improvements in cancer-related fatigue, although the
mechanisms for these effects have not been determined. There is also compelling
evidence that psychosocial interventions may improve energy and other aspects
of mental and physical function in cancer patients. Few studies have examined
pharmacologic treatments for cancer-related fatigue other than erythropoietin;
however, preliminary evidence suggests that psychostimulants may be effective
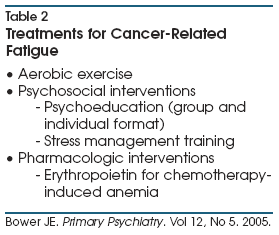
for patients with advanced cancer (Table 2).
Conclusion
The growing literature on
cancer-related fatigue highlights the prevalence of this symptom and its
importance for quality of life in cancer patients and survivors. Fatigue in
cancer patients is likely multifactorial and influenced by physiological
changes associated with the disease and/or its treatment, comorbid medical
conditions, physical symptoms, and psychological responses to the cancer
experience. Fatigue is known to co-occur with sleep disturbance, pain, and
particularly depression in cancer patients.
The interrelationship
between fatigue and depression is complex: fatigue may be a symptom of
depression, may precipitate feelings of depression, or they may both be
symptoms of an underlying biological disturbance. The literature indicates that
cancer-related fatigue cannot be explained by depression and that treatment
with paroxetine has differential effects on depression and fatigue in cancer
patients undergoing treatment, suggesting different biological mechanisms.
There is limited research on biological correlates of
fatigue, although preliminary evidence suggests that fatigue may be associated
with an inflammatory process in cancer patients and survivors. Alterations in
HPA axis function observed in fatigued breast cancer survivors may be a cause
or consequence of these immune alterations. Investigators are developing
targeted treatments for cancer-related fatigue, including exercise,
psychosocial interventions, and pharmacotherapy. All of these therapeutic
modalities have been associated with improvements in cancer-related fatigue,
with the strongest and most consistent results seen in trials utilizing aerobic
exercise. PP
References
1. Ries LAG, Eisner MP, Kosary CL, et al, eds.Surveillance, Epidemiology, and End
Results Cancer Statistics Review, 1975–2000. Bethesda, MD: National Cancer Institute; 2003.
2. Wagner LI, Cella D. Fatigue and cancer: causes,
prevalence and treatment approaches. Br
J Cancer. 2004;91(5):822-828.
3. Irvine DM, Vincent
L, Graydon JE, Bubela N. Fatigue in women with breast cancer receiving
radiation therapy. Cancer Nurs.
1998;21(2):127-135.
4. Hann DM, Jacobsen
PB, Azzarello LM, et al. Measurement of fatigue in cancer patients: development
and validation of the Fatigue Symptom Inventory. Qual Life Res. 1998;7(4):301-310.
5. Vogelzang NJ, Breitbart W, Cella D, et al, for the
Fatigue Coalition. Patient, caregiver, and oncologist perceptions of
cancer-related fatigue: results of a tripart assessment survey. Semin Hematol. 1997;34(3
suppl 2):4-12.
6. Bower JE, Ganz PA, Desmond KA, Rowland JH, Meyerowitz
BE, Belin TR. Fatigue in breast cancer survivors: occurrence, correlates, and
impact on quality of life. J
Clin Oncol. 2000;18(4):743-753.
7. Loge JH, Abrahamsen AF, Ekeberg O, Kaasa S. Hodgkin’s
disease survivors more fatigued than the general population. J Clin Oncol.
1999;17(1):253-261.
8. Broeckel JA, Jacobsen PB, Horton J, Balducci L, Lyman
GH. Characteristics and correlates of fatigue after adjuvant chemotherapy for
breast cancer. J Clin Oncol.
1998;16(5):1689-1696.
9. Poulson MJ. Not just tired. J Clin Oncol. 2003;21(suppl
9):112-113.
10. Cella D, Peterman A, Passik S, Jacobsen P, Breitbart W.
Progress toward guidelines for the management of fatigue. Oncology (Huntingt).
1998;12(11A):369-377.
11. Glaus A. Fatigue in patients with cancer. Analysis and
assessment. Recent Results
Cancer Res. 1998;145:1-172.
12. Andrykowski MA,
Curran SL, Lightner R. Off-treatment fatigue in breast cancer survivors: a
controlled comparison. J Behav Med.
1998;21(1):1-18.
13. Mast ME. Correlates
of fatigue in survivors of breast cancer. Cancer Nurs. 1998;21(2):136-142.
14. Jacobsen PB, Stein K. Is fatigue a long-term side
effect of breast cancer treatment? Cancer
Control. 1999;6(3):256-263.
15. Holzner B, Kemmler G, Greil R, et al. The impact of
hemoglobin levels on fatigue and quality of life in cancer patients. Ann Oncol.
2002;13(6):965-973.
16. Cella D, Kallich J, McDermott A, Xu X. The longitudinal
relationship of hemoglobin, fatigue and quality of life in anemic cancer
patients: results from five randomized clinical trials. Ann Oncol.
2004;15(6):979-986.
17. Geinitz H, Zimmermann FB, Stoll P, et al. Fatigue,
serum cytokine levels, and blood cell counts during radiotherapy of patients
with breast cancer. Int J
Radiat Oncol Biol Phys. 2001;51(3):691-698.
18. Cella D, Lai JS, Chang CH, Peterman A, Slavin M.
Fatigue in cancer patients compared with fatigue in the general United States population. Cancer.
2002;94(2):528-538.
19. Wang XS, Giralt SA, Mendoza TR, et al. Clinical factors
associated with cancer-related fatigue in patients being treated for leukemia
and non-Hodgkin’s lymphoma. J
Clin Oncol. 2002;20(5):1319-1328.
20. Dantzer R. Cytokine-induced sickness behavior:
mechanisms and implications. Ann
N Y Acad Sci. 2001;933:222-234.
21. Denicoff KD, Rubinow DR, Papa MZ, et al. The
neuropsychiatric effects of treatment with interleukin-2 and
lymphokine-activated killer cells. Ann
Intern Med. 1987;107(3):293-300.
22. Spath-Schwalbe E, Hansen K, Schmidt F, et al. Acute
effects of recombinant human interleukin-6 on endocrine and central nervous
sleep functions in healthy men. J
Clin Endocrinol Metab. 1998;83(5):1573-1579.
23. Reichenberg A, Yirmiya R, Schuld A, et al.
Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry.
2001;58(5):445-452.
24. Greenberg DB, Gray JL, Mannix CM, Eisenthal S, Carey M.
Treatment-related fatigue and serum interleukin-1 levels in patients during
external beam irradiation for prostate cancer. J Pain Symptom Manage. 1993;8(4):196-200.
25. Mills PJ, Aadler KA, Perez CJ, et al. Soluble ICAM-1
and fatigue in breast cancer patients in response to chemotherapy. Abstract
presented at: Annual Scientific Meeting of the American Psychosomatic Society;
March 5–8, 2003; Phoenix, AZ.
26. Bower JE, Ganz PA, Aziz N, Fahey JL. Fatigue and
proinflammatory cytokine activity in breast cancer survivors. Psychosom Med.
2002;64(4):604-611.
27. Bower JE, Ganz PA, Aziz N, Fahey JL, Cole SW. T-cell
homeostasis in breast cancer survivors with persistent fatigue. J Natl Cancer Inst.
2003;95(15):1165-1168.
28. McEwen BS, Biron CA, Brunson KW, et al. The role of
adrenocorticoids as modulators of immune function in health and disease:
neural, endocrine and immune interactions. Brain
Res Brain Res Rev. 1997;23(1-2):79-133.
29. Demitrack MA, Crofford LJ. Evidence for and
pathophysiologic implications of hypothalamic-pituitary-adrenal axis
dysregulation in fibromyalgia and chronic fatigue syndrome. Ann N Y Acad Sci.
1998;840:684-697.
30. Bower JE, Ganz PA, Dickerson SS, Petersen L, Aziz N,
Fahey JL. Diurnal cortisol rhythm and fatigue in breast cancer survivors. Psychoneuroendocrinology.
2005;30(1):92-100.
31. Bower JE, Ganz PA, Aziz N. Altered cortisol response to
psychologic stress in breast cancer survivors with persistent fatigue. Psychosom Med.
2005;67(2):277-280.
32. Jacobsen PB, Donovan KA, Weitzner MA. Distinguishing
fatigue and depression in patients with cancer. Semin Clin Neuropsychiatry. 2003;8(4):229-240.
33. Massie MJ. Prevalence of depression in patients with cancer.
J Natl Cancer Inst Monogr.
2004;(32):57-71.
34. Visser MR, Smets
EM. Fatigue, depression and quality of life in cancer patients: how are they
related? Support Care Cancer.
1998;6(2):101-108.
35. Musselman DL, Miller AH, Porter MR, et al. Higher than
normal plasma interleukin-6 concentrations in cancer patients with depression:
preliminary findings. Am J
Psychiatry. 2001;158(8):1252-1257.
36. Morrow GR, Andrews PL, Hickok JT, Roscoe JA, Matteson
S. Fatigue associated with cancer and its treatment. Support Care Cancer. 2002;10(5):389-398.
37. Morrow GR, Hickok
JT, Roscoe JA, et al, for the University of Rochester Cancer Center Community
Clinical Oncology Program. Differential effects of paroxetine on fatigue and
depression: a randomized, double-blind trial from the University of Rochester Cancer Center Community Clinical Oncology Program. J Clin Oncol. 2003;21(24):4635-4641.
38. Musselman DL, Lawson DH, Gumnick JF, et al. Paroxetine
for the prevention of depression induced by high-dose interferon alfa. N Engl J Med.
2001;344(13):961-966.
39. Valentine AD, Meyers CA, Kling MA, Richelson E, Hauser
P. Mood and cognitive side effects of interferon-alpha therapy. Semin Oncol. 1998;25(1 suppl
1):39-47.
40. Capuron L, Gumnick
JF, Musselman DL, et al. Neurobehavioral effects of interferon-alpha in cancer
patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology. 2002;26(5):643-652.
41. Mock V. Evidence-based treatment for cancer-related
fatigue. J Natl Cancer Inst
Monogr. 2004;(32):112-118.
42. Courneya KS, Mackey JR, Bell GJ, Jones LW, Field CJ, Fairey AS. Randomized controlled trial of exercise training in postmenopausal breast cancer
survivors: cardiopulmonary and quality of life outcomes. J Clin Oncol.
2003;21(9):1660-1668.
43. Helgeson VS, Cohen S, Schulz R, Yasko J. Education and
peer discussion group interventions and adjustment to breast cancer. Arch Gen Psychiatry.
1999;56(4):340-347.
44. Helgeson VS, Cohen
S, Schulz R, Yasko J. Long-term effects of educational and peer discussion
group interventions on adjustment to breast cancer. Health Psychol. 2001;20(5):387-392.
45. Fawzy FI, Cousins N, Fawzy NW, Kemeny ME, Elashoff R,
Morton D. A structured psychiatric intervention for cancer patients. I. Changes
over time in methods of coping and affective disturbance. Arch Gen Psychiatry.
1990;47(8):720-725.
46. Spiegel D, Bloom JR, Yalom I. Group support for
patients with metastatic cancer. A randomized outcome study. Arch Gen Psychiatry. 1981;38(5):527-533.
47. Given B, Given CW, McCorkle R, et al. Pain and fatigue
management: results of a nursing randomized clinical trial. Oncol Nurs Forum.
2002;29(6):949-956.
48. Jacobsen PB, Meade
CD, Stein KD, Chirikos TN, Small BJ, Ruckdeschel JC. Efficacy and costs of two
forms of stress management training for cancer patients undergoing
chemotherapy. J Clin Oncol.
2002;20(12):2851-2862.
49. Sarhill N, Walsh D, Nelson KA, Homsi J, LeGrand S,
Davis MP. Methylphenidate for fatigue in advanced cancer: a prospective
open-label pilot study. Am J
Hosp Palliat Care. 2001;18(3):187-192.
50. Bruera E, Driver L, Barnes EA, et al.
Patient-controlled methylphenidate for the management of fatigue in patients
with advanced cancer: a preliminary report. J
Clin Oncol. 2003;21(23):4439-4443.