Electroconvulsive Therapy in
Treatment-Resistant Depression
Sanjay J. Mathew, MD, Jonathan
M. Amiel, BS,
and Harold A. Sackeim, PhD
Dr. Mathew is assistant
professor of psychiatry in the Department of Psychiatry at Mount Sinai School
of Medicine in New York City.
Mr. Amiel is
a medical student at Columbia University College of Physicians and Surgeons in New York City.
Dr. Sackeim
is professor of psychiatry and radiology in the Departments of Psychiatry and
Radiology at Columbia University College of Physicians and Surgeons, and chief
of the Department of Biological Psychiatry at the New York State Psychiatric
Institute in New York City.
Disclosure:
The authors report no financial, academic, or other interest in any
organization that may pose a conflict of interest.
Funding/support:
This work was supported in part by National Institute of Mental Health Career
Development Award MH069656 given to Dr. Mathew.
Please direct all correspondence to: Sanjay J. Mathew, MD,
Department of Psychiatry, Mount Sinai School of Medicine, One Gustave Levy
Place, Box 1218, New York, NY 10029; Tel: 212-241-4480; Fax: 212-860-3369;
E-mail: [email protected].
Focus Points
• Electroconvulsive
therapy (ECT) is the most effective form of treatment for patients with
treatment-resistant depression.
• Relapse after ECT is
commonly observed. The combination of lithium and nortriptyline has been shown
to reduce the rates of relapse.
• While bilateral ECT
is commonly administered in the community, newer modes of unilateral ECT have
been developed that are equal in efficacy to bilateral ECT and have less
cognitive side effects.
Abstract
What is the role of
electroconvulsive therapy (ECT) in the treatment of mood disorders, especially
treatment-resistant depression (TRD)? While many have thought that ECT has been
replaced by pharmacotherapy, ECT is still frequently used, with the number of
treatments conducted annually in the United States exceeding coronary bypass,
appendectomy, tonsillectomy, and hernia repair. Controlled studies and clinical
experience indicate that the short-term efficacy of ECT in major depression is
comparable, and likely superior, to that of any other antidepressant treatment.
Patients with TRD consume a disproportionate share of medical resources and
this condition often presents extraordinary burdens to the individual, family,
and society. Relative to any other antidepressant treatments, ECT has the
highest rate of response/remission, the fastest onset of symptom relief, and
the most complete symptom relief. However, ECT has two major drawbacks that
limit its use: its effects on memory and high rate of relapse. Current research
is directed at improving cognitive outcomes and identifying more effective
prophylactic treatment following ECT. Novel innovations include methods to
produce seizures with focal onset and limited propagation. Such modifications
have the potential to markedly reduce the adverse effects of ECT without
compromising efficacy.
Introduction
Electroconvulsive therapy
(ECT) is the oldest, continuously practiced biological intervention in
psychiatry. It remains our most effective antidepressant treatment despite
considerable progress in the pharmacologic treatment of major depression.1,2
There was a sharp decline in ECT use in the United States following the
introduction of pharmacologic agents to treat depression in the 1950s. In the
late 1980s, the utilization rate of ECT stabilized and current estimates are
that approximately 100,000 individuals receive ECT in the US each year, with an
annual rate of 1 million–2 million individuals receiving ECT worldwide.3
ECT is usually administered to patients with severe and medication-resistant
major depression, as
well as mania, catatonia, and acute exacerbations of schizophrenia.4 ECT involves a series of
treatments, usually administered at a rate of 2–3/week, with most individuals
with major depression requiring 6–12 treatments overall to achieve full remission.
Thus, nearly 1 million ECT procedures are performed in the US annually and is performed as commonly as tonsillectomy.3
All comparator studies have found ECT equal or superior to
antidepressant medications in the extent of short-term clinical improvement,
with remission rates following ECT on the order of 70% to 90%.5-7
ECT remains the most effective intervention for treatment-resistant depression
(TRD). Factors in its favor include that speed of clinical improvement is
typically quicker with ECT and that the quality of symptomatic remission is
better (ie, fewer residual symptoms). The latter is important, in part, because
there is consistent evidence that the severity of residual symptoms following
any intervention for major depression is predictive of relapse.1
Despite its strong and unique short-term efficacy, there are two major
limitations that restrict the use of ECT: cognitive side effects and the high
rate of relapse after response. The magnitude and persistence of cognitive side
effects is strongly related to technical factors in how ECT is performed, but
also varies considerably among individuals. Notably, a small proportion of
former ECT patients report profound and persistent cognitive side effects,
where objective correlates are sometimes uncertain. Regarding relapse after
ECT, optimal methods of prevention have yet to be identified, particularly for
TRD.
This review summarizes the clinical indications for ECT and describes
the pre-ECT medical and psychiatric workups, with a review of the systematic
evaluation of TRD using the Antidepressant Treatment History Form (ATHF). The
complex relationship between medication resistance and the short-term response
to ECT is reviewed, as well as the evidence regarding predictors of response to
ECT. For primary care physicians (PCPs) or psychiatrists treating patients
following a course of ECT, guidelines are provided for continuation and
maintenance treatment based on the empirical literature. New research findings
are discussed in the optimization of treatment technique for ECT, with a brief
summary of the decades-long controversy regarding the relative efficacy of
bilateral versus unilateral ECT. Finally, recent innovations in ECT treatment
technique that aim to ameliorate the cognitive side-effect burden are
discussed.
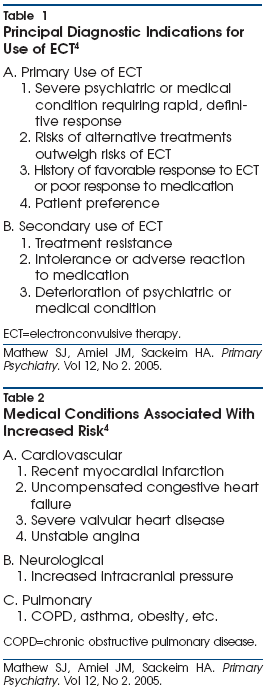
Indications for Electroconvulsive Therapy
PCPs and psychiatrists treating patients with major depression are faced
with a number of options for treatment and referral. The recommendation of ECT
is informed by issues of clinical urgency, particularly the severity of
symptoms, including suicidal intent, inanition, psychosis, and marked agitation
or retardation. These factors must be weighed together with the patient’s
medical condition (eg, cardiovascular disease), previous history of side
effects on pharmacologic regimens and ECT, previous history of ECT outcomes
(cognitive and therapeutic), urgency of life circumstances, and patient
preference (Table 1). For example, ECT is particularly useful for patients who
are acutely suicidal and hard-pressed to wait several weeks for antidepressants
to become effective or for patients who do not tolerate antidepressants. When
the rationale for ECT is not compelling, alternative medication strategies are
recommended.
While much of the focus for the indications has been on TRD, a variety
of factors may lead to the recommendation to use ECT as a first-choice
treatment. For example, in depressed pregnant patients, ECT has proven efficacy
and is safer for both mother and fetus than some pharmacologic alternatives.
ECT may be used in pregnant women during all three trimesters because the risks
of alternative pharmacologic treatment or untreated mental illness exceed those
of ECT.4
One of the most significant barriers to referral for ECT remains the
degree of stigma regarding the procedure in the general population and among
medical professionals. Medical students generally have little exposure to or
training in ECT, though they commonly express a bias against the procedure.8
Similar ambivalence in the professional community is evident in the significant
variability in regional availability of ECT and in the lack of ECT research in
many major psychiatric research centers.9,10 Interestingly, the
majority of patients who have undergone ECT would be willing to go through
treatment again, and many consider a trip to the dentist more distressing.11-13
Pre-Electroconvulsive
Therapy Evaluation
There are no absolute
contraindications for ECT. In fact, ECT is often used in medically compromised
patients due to its rapid therapeutic onset and relative safety.4 All patients should undergo
thorough pre-ECT evaluation including psychiatric history, medical history,
blood work-up (complete blood count, serum chemistries, thyroid-stimulating
hormone, liver function tests), and electrocardiogram. In patients >50 years
of age or with compromised dentition, a dental assessment is indicated due to
stimulation of jaw musculature by ECT. While not required in all patients,
brain imaging studies (magnetic resonance imaging and/or computed tomography
scan) may be indicated in patients with a sudden, precipitous change in mental
status, or who have notable cardiovascular and cerebrovascular risk factors.
Medical histories of cardiovascular, neurological, or pulmonary diseases
should be emphasized as they warrant special care in anesthesia and may also
dictate modifications of ECT technique (Table 2). Medication may be
administered to reduce treatment-related hypertension, and additional
physiological monitoring during the ECT procedure may also be indicated. In
addition, patients starting an ECT course may continue treatment with
nondiuretic antihypertensives, antianginals, nonlidocaine antiarrhythmics,
antireflux agents, nontheophylline bronchodilators, nonacetylcholinesterase
glaucoma medications, and corticosteroids. Diuretic and hypoglycemic medications
should be withheld until after each treatment. The concurrent use of
psychotropic medications during a course of ECT is controversial. For
benzodiazepines, which have the potential to reduce seizure duration and
expression, it has been shown that lorazepam, in the range of 0–3 mg/day and
withheld at least 10 hours before ECT, has no relation to seizure threshold.14
Assessment of Treatment-Resistant Depression
A diagnosis of TRD must satisfy two conditions: the patient had
insufficient benefit from pharmacologic antidepressant treatment and the
pharmacologic treatments established efficacy for major depression and were
administered at sufficient dosage and duration. While standardized measures for
assessing depressive symptoms, such as the Hamilton Rating Scale for Depression
(HAM-D)15 and the Beck Depression Inventory,16 have been
available for many years, there have been few attempts to formally assess the
adequacy of pharmacologic treatment. However, in recent years substantial
evidence has emerged indicating that many patients with major depression never
receive treatment trials of adequate dosage and/or duration.17-19
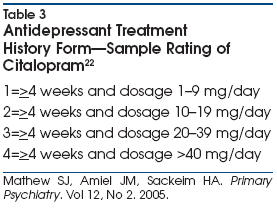
The ATHF is one of the
broadest and most commonly used clinician-rated instruments for evaluating the
adequacy of prior treatments in TRD.20,21 The ATHF rates each
pharmacologic intervention on a five-point scale, with lower scores given to
antidepressants administered at inadequate dosage or for <4 weeks.
Regardless of dose and duration, a low score (£2)
is given to interventions without established efficacy for major depression.
Specifically, positive findings from randomized, placebo-controlled trials must
be available for an intervention to be considered an adequate treatment. Higher
ATHF scores are assigned to antidepressant treatments lasting >4
weeks at doses that meet threshold levels or higher for adequacy. Specifically,
the ATHF levels for considering a trial adequate (ratings of 3 or above) use a
threshold dosage equal to the dosage used in randomized clinical trials that
demonstrated the efficacy of the agent in major depression.22 An
example of ATHF criteria for the commonly prescribed antidepressant citalopram
is illustrated in Table 3.22
What are the Factors that Impinge on the Short-Term Outcome
of Electroconvulsive Therapy?
Work on the prediction of response to ECT has centered on phenomenology,
clinical history, or neurobiological measures. In patients that presently
receive ECT, clinical features have shown surprisingly little relation to
clinical outcome.23 The presence or absence of subtypes of major
depression such as melancholia and bipolar depression do not have predictive
value regarding likelihood of response to ECT. On the other hand, one factor
that portends a relatively poorer outcome following ECT is the presence of axis
II comorbidity, particularly borderline or narcissistic personality disorders.24
There has been some
research suggesting that patients with psychotic depression (or delusional
depression) have a superior response rate relative to nonpsychotic depressed
patients.25-27 However, this association is likely due to the low
rate of medication resistance in patients referred to ECT with psychotic
depression.28,29 Optimal pharmacotherapy for psychotic depression
requires combination treatment with an antidepressant and antipsychotic
medication. Even with the introduction of atypical antipsychotics, this
combination is rarely administered to patients with psychotic depression at
adequate dosage. In particular, due to problems of tolerability, patients with
psychotic depression rarely receive adequate dosage of the antipsychotic
medication. Consequently, the relative lack of medication resistance among
patients with psychotic depression may account for their seeming preferential
response to ECT. Treatment history has rarely been evaluated as a predictor of
response to subsequent antidepressant treatment, although there are now data
suggesting that this factor might be critical to prognosis. Various researchers30-32
have repeatedly shown that medication-resistant patients have a substantially
lower rate of ECT response. Approximately 90% of the patients who
have not received an adequate medication trial during the current episode will
meet remission criteria when treated with ECT. This rate is reduced to
approximately 50% in medication-resistant patients. While medication resistance
substantially impacts on efficacy, ECT may nonetheless still have the highest
probability of producing remission, as the likelihood of benefit is often lower
with pharmacologic alternatives.
Relapse Prevention:
What to Do After Electroconvulsive Therapy
From the perspective of the referring PCP or psychiatrist, post-ECT
care is a critical issue. Optimization of follow-up care begins during the ECT
course, when regular communication between the ECT team and the referring
physician is vital, especially if ECT has been conducted on an inpatient basis.
When the patient is discharged from the ECT facility, the referring physician
should request a summary of the ECT course, including number of treatments,
type of treatment (unilateral versus bilateral), pulse width, type of
anesthesia, medications administered, documentation of endpoint cognitive
status, and formal assessment of depression. Unfortunately, such formal
assessments and communication are rarely performed in non-research settings.
Patients are often discharged from ECT facilities without appropriate
continuation medication, and are given only a general statement to the effect
that they are “doing much better.”
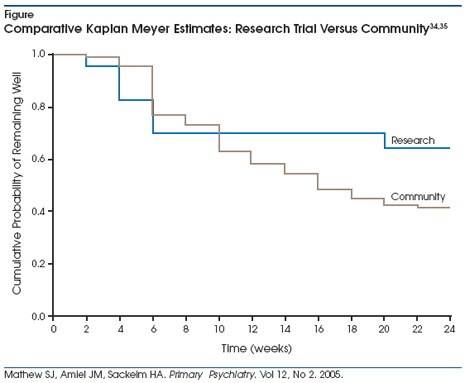
Not surprisingly, relapse
following ECT response is common.33 Without active treatment,
virtually all remitted patients will relapse within 6 months following ECT,
although post-ECT therapy with nortriptyline and lithium reduces relapse rates
to approximately 40%.34 Virtually all cases of relapse with this
combination occur within 5 weeks of the end of ECT. In the community, relapse
rates are higher than in the research setting, even if the patient achieved
remission at the end of ECT (Figure).34,35 Higher post-ECT relapse
rates in community settings are likely due to premature termination of ECT by
practitioners unaware of the extent to which improvement is incomplete. Though
practitioners in the community typically terminate ECT treatment when they
believe maximal therapeutic benefit has been achieved, patients often show
considerable residual symptoms at termination.
The preponderance of early
relapse may be expected, as standard practice involves abrupt discontinuation
of ECT once it is effective. Since antidepressants generally take several weeks
before symptomatic improvement is observed, patients are left vulnerable in the
first several weeks following a standard switch from ECT to continuation
pharmacotherapy. Continuation therapy with nortriptyline and lithium has shown
a markedly lower relapse rate compared to nortriptyline alone or placebo alone
in the 6 months following ECT.34 These findings require replication
with different combinations of antidepressants and lithium. The same group is
now investigating whether beginning an antidepressant at ECT initiation
provides a “head start” and protects against early relapse. The role of
continuation ECT as part of the post-acute regimen is being studied in certain
patients. Preliminary data from a large, randomized, prospective study
comparing the lithium/nortriptyline combination versus continuation ECT has
suggested a superior relapse-free interval in the medication group.
The authors recommend the
early use of continuation medication treatment in patients who have completed a
course of ECT, whether they are remitters, partial responders, or
nonresponders. While the combination of lithium and nortriptyline for post-ECT
treatment has the most solid research backing, and appears to be more effective
than continuation ECT, other combinations, eg, lithium-venlafaxine extended
release, are currently being tested in large-scale studies.
Innovations in
Electroconvulsive Therapy Administration and Technique
In 1980, the view of ECT was that its efficacy depended only on the
generalized seizure, while cognitive side effects were determined by the
intensity of electrical stimulation. A series of studies5,6 at Columbia University has demonstrated that the efficacy of right unilateral (RUL) ECT is
highly dependent on the degree to which dosage exceeded seizure threshold (ST).
It was recently demonstrated that high dosage RUL ECT matches the efficacy of a
robust form of bilateral (BL) ECT while maintaining advantages in acute, short-
and long-term cognitive effects.36 These findings have helped
resolve the controversy in the field about the relative merits of RUL and BL
ECT.
A more recent innovation
involves the narrowing of pulse width (PW). The traditional ECT stimulus is
nonphysiologic, depositing energy long after depolarization.37 A new
study indicates that ultra brief (UB) stimulation is far more efficient in
seizure induction, lowering seizure threshold by a factor of 3–4. Critically,
the differences between wide PW (1.5 ms) and UB (0.3 ms) PW in cognitive side
effects often exceeded the differences between RUL and BL ECT. Given its
efficacy and cognitive sparing, UB stimulation may soon become the standard in
the field.
Finally, a limitation of current ECT technique is the lack of control
over intracerebral current paths and current density. High skull impedance and
variation in skull anatomy result in shunting of the stimulus away from the
brain with uncontrolled variation in intracerebral spatial distribution. In
particular, seizure propagation in medial temporal lobe regions is believed to
contribute to the cognitive side effects of ECT, while not contributing to
efficacy. The authors are now studying the feasibility of focal
electrically-administered seizure therapy (FEAST). Recently, seizures have been
elicited in nonhuman primates at a very low dosage (3 mC), which were expressed
in frontal electroencephalogram, did not propagate to motor cortex, and did not
result in motor convulsions. It is expected that FEAST’s spatial targeting of
prefrontal cortex, with reduced involvement of medial temporal lobe, will
preserve efficacy and reduce amnestic side effects. Controlled investigations
are in progress.
Conclusion
The practice of ECT remains an important option for patients with TRD,
as well as for particular patients who are not treatment resistant. While the
efficacy of ECT is comparable to any other intervention and generally surpasses
pharmacotherapy in TRD, high relapse rates and cognitive side effects have
limited its potential broader applicability. Priorities for future
investigation include identification of optimal continuation and maintenance
treatments following ECT (whether pharmacologic, psychosocial, or further ECT),
optimization of ECT treatment technique to enhance efficacy and limit cognitive
side effects, and identification of biomarkers that might predict therapeutic
response to treatment. As one example of this direction, the use of
non-invasive neuroimaging techniques such as proton magnetic resonance
spectroscopy (MRS) would enable the physician to quantify cerebral g-aminobutyric acid (GABA) prior to treatment.
Obtaining serial MRS examinations during the course of ECT would allow
quantification and correlation with improvements in depression scores. Instead
of simply stating that the patient appears “much better” and discharging from
ECT, the treatment team might follow HAM-D scores to remission, while
scrutinizing for normalization of GABA. A failure of ECT to normalize brain
GABA might indicate that further treatment is necessary, although the patient
might appear clinically improved. Future investigations that integrate multiple
modes of inquiry (genetics, neuroimaging, clinical phenomenology) are needed to
answer fundamental questions regarding the role of ECT in patients with
difficult-to-treat mood disorders. PP
References
1. Sackeim HA,
Devanand DP, Nobler MS. Electroconvulsive therapy. In: Bloom FE, Kupfer DJ,
eds. Psychopharmacology: The
Fourth Generation of Progress. New York, NY: Raven Press, Ltd;
1995:1123-1141.
2. Sackeim HA, Prohovnik I, Moeller JR, et al. Regional cerebral
blood flow in mood disorders. I. Comparison of major depressives and normal
controls. Arch Gen Psychiatry.
1990;47:60-70.
3. Thompson JW, Weiner RD, Myers CP. Use of ECT in the United States in 1975, 1980, and 1986. Am J Psychiatry.
1994;151:1657-1661.
4. American
Psychiatric Association. The
Practice of Electroconvulsive Therapy: Recommendations for Treatment, Training,
and Privileging. 2nd ed. Washington, DC: American Psychiatric
Association; 2001.
5. Sackeim HA, Prudic
J, Devanand DP, et al. Effects of stimulus intensity and cognitive effects of
electroconvulsive therapy. N
Engl J Med. 1993;328:839-846.
6. Sackeim HA, Prudic J, Devanand DP, et al. A prospective,
randomized, double-blind comparison of bilateral and right unilateral
electroconvulsive therapy at different stimulus intensities. Arch Gen
Psychiatry. 2000;57:425-434.
7. Petrides G, Fink M,
Husain MM, et al. ECT remission rates in psychotic versus nonpsychotic
depressed patients: A report from CORE. J
ECT. 2001;17:244-253.
8. Clothier JL,
Freeman T. Snow L. Medical student attitudes and knowledge about ECT. J ECT. 2001;17:99-101.
9. Salzman C. ECT,
research, and professional ambivalence. Am
J Psychiatry. 1998;155:1-2.
10. Hermann RC, Dorwart
RA, Hoover CW, Brody J. Variation in ECT use in the United States. Am J Psychiatry.
1995;152:869-875.
11. Hughes J,
Barraclough BM, Reeve W. Are patients shocked by ECT? J Royal Soc Med.
1981;74:283-285.
12. Pettinati HM,
Tamburello TA, Ruetsch CR, Kaplan FN. Patient attitudes towards
electroconvulsive therapy. Psychopharmacol
Bull. 1994;30:471-575.
13. Goodman JA, Krahn
LE, Smith GE, et al. Patient satisfaction with electroconvulsive therapy. Mayo Clin Proceed.
199;74:967-971.
14. Boylan LS, Haskett
RF, Mulsant BH, et al. Determinants of seizure threshold in ECT: benzodiazepine
use, anesthetic dosage, and other factors. J
ECT. 2000;16:3-18.
15. Hamilton M.
Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol.
1967;6:278-296.
16. Beck AT, Steer RA,
Garbin MG. Psychometric properties of the Beck Depression Inventory: twenty
five years of evaluation. Clin
Psychol Rev 8. 1988;1:77-100.
17. Keller MB, Lavori PW, Klerman GL, et al. Low levels and lack of
predictors of somatotherapy and psychotherapy received by depressed patients. Arch Gen
Psychiatry. 1986;43:458-466.
18. Keller MB, Klerman
GL, Lavori PW, et al. Treatment received by depressed patients. JAMA. 1982;248:1848-1855.
19. Dawson R, Lavori PW,
Coryell WH, et al. Course of treatment received by depressed patients. J Psychiatr Res.
1999;33:233-242.
20. Prudic J, Haskett
RF, Mulsant B, et al. Resistance to antidepressant medications and short-term
clinical response to ECT. Am J
Psychiatry. 1996;153:985-992.
21. Mulsant BH, Haskett
RF, Prudic J, et al. Low use of neuroleptic drugs in the treatment of psychotic
major depression. Am J
Psychiatry. 1997;154:559-561.
22. Sackeim HA. The
definition and meaning of treatment-resistant depression. J Clin Psychiatry.
2001;62(Suppl 16):10-17.
23. Nobler
MS, Sackeim HA. Electroconvulsive therapy: clinical and biological aspects. In:
Goodnick PJ, ed. Predictors of
Response in Mood Disorders. Washington, DC: American Psychiatric
Press; 1996:177-198.
24. DeBattista C,
Mueller K. Is electroconvulsive therapy effective for the depressed patient
with comorbid borderline personality disorder?
J ECT. 2001;17:91-98.
25. Buchan H, Johnstone
E, McPherson K, Palmer RL, Crow TJ, Brandon S. Who benefits from
electroconvulsive therapy? Combined results of the Leicester and Northwick Park trials. Br J Psychiatry.
1992;160:355-359.
26. Brandon S, Cowley P,
McDonald C, et al. Electroconvulsive therapy: results in depressive illness
from the Leicestershire trial. Br
Med J. 1984;288:22-25.
27. Janicak
PG, Easton MS, Comaty JE, Dowd S, Davis JM. Efficacy of ECT in psychotic and
nonpsychotic depression. Convulsive
Ther. 1989;5:314-320.
28. Prudic J,
Sackeim HA, Devanand DP. Medication resistance and clinical response to
electroconvulsive therapy. Psychiatry
Res. 1990;31:287-296.
29. Mulsant BH, Haskett
RF, Prudic J, et al. Low use of neuroleptic drugs in the treatment of psychotic
major depression. Am J
Psychiatry. 1997;154:559-561.
30. Flint AJ, Rifat SL.
The effect of sequential antidepressant treatment on geriatric depression. J Affect Disord.
1996;36:95-105.
31. Prudic J, Haskett
RF, Mulsant B, et al. Resistance to antidepressant medications and short-term
clinical response to ECT. Am J
Psychiatry. 1996;153:985-992.
32. Quitkin F. The
importance of dosage in prescribing antidepressants. Br J Psychiatry. 1985;147:593-597.
33. Sackeim HA.
Continuation therapy following ECT: directions for future research. Psychopharmacol Bull.
1994;30:501-521.
34. Sackeim HA, Haskett
RF, Mulsant BH, et al. Continuation pharmacotherapy in the prevention of
relapse following electroconvulsive therapy. JAMA.
2001;285:1299-1307.
35. Prudic J,
Olfson M, Marcus SC, Fuller RB, Sackeim HA. Effectiveness of electroconvulsive
therapy in community settings. Biol
Psychiatry. 2004;55:301-312.
36. Sackeim HA, Prudic J, Devanand DP, et al. A prospective,
randomized, double-blind comparison of bilateral and right unilateral
electroconvulsive therapy at different stimulus intensities. Arch Gen
Psychiatry. 2000;57:425-434.
37. Sackeim HA, Long
J, Luber B, et al. Physical properties and quantification of the ECT stimulus:
I. Basic principles. Convuls
Ther. 1994;10:93-123.