Focal Brain Stimulation For Treatment–Resistant
Depression: Transcranial Magnetic Stimulation, Vagus-Nerve Stimulation, and
Deep-Brain Stimulation
Paul E. Holtzheimer III, MD and David H. Avery, MD
Dr. Holtzheimer is assistant professor of psychiatry in the
Department of Psychiatry and Behavioral Sciences at Emory University in Atlanta, Georgia.
Dr.
Avery is professor of psychiatry in the Department of Psychiatry and Behavioral
Sciences at University of Washington School of Medicine in Seattle, Washington.
Disclosure: The authors report no
financial, academic, or other interest in any organization that may pose a
conflict of interest. Drs. Holtzheimer and Avery are currently co-investigators
in a multi-site study of repetitive transcranial magnetic stimulation sponsored
by Neuronetics, Inc.
Funding/support: This work was supported
in part by the National Institute of Mental Health, grant no. R01 MH062154
awarded to Dr. Avery.
Please direct all correspondence to: David H. Avery, MD,
Department of Psychiatry and Behavioral Sciences, Harborview Medical Center,
325 Ninth Ave, Box 359896, Seattle, WA 98104; Tel: 206-731-4527; Fax:
206-731-8615; E-mail: [email protected].
Focus points
•
Interest in focal brain stimulation has largely arisen from an attempt to find
novel treatments for depression with similar efficacy as electroconvulsive
therapy (ECT) but without the risks and side effects associated with ECT.
• Transcranial magnetic
stimulation (TMS) has a large database supporting statistically significant
antidepressant effects; however, the clinical significance of these effects has
yet to be established.
• Certain patient- and
treatment-specific factors may have a profound influence on the antidepressant
effects seen with TMS; the clinical relevance of TMS for treatment-resistant
depression (TRD) may be demonstrated by large studies paying careful attention
to these factors.
• Vagus-nerve stimulation has
mixed data supporting its efficacy for TRD, but long-term follow-up data
suggest it may indeed have clinically important antidepressant effects in at
least some patients.
• Deep-brain stimulation (DBS)
has not yet shown efficacy in TRD; however, known effects of DBS on mood in
some patients and its ability to directly modulate function of subcortical
structures, make it an excellent candidate as a novel treatment for TRD.
• Although it is unclear what role they will eventually play in the treatment of
TRD, focal brain stimulation techniques offer an exciting new way to modulate
function in brain areas involved in mood regulation and depression.
Abstract
There is an increased
interest in focal brain stimulation as a potential treatment for depression.
This is largely due to an attempt to find treatments for depression that have
efficacy similar to electroconvulsive therapy (ECT) but are not associated with
the same limitations as ECT. This article describes three techniques for focal
brain stimulation: transcranial magnetic stimulation, vagus-nerve stimulation,
and deep-brain stimulation. It also discusses the data supporting their use in
treatment-resistant depression.
Introduction
Major depression is a
common and disabling illness associated with increased mortality, both from
suicide and nonsuicidal causes.1,2 Fortunately, most depressed
patients will respond adequately to medications and/or psychotherapy; however,
there are a number of patients who do not respond to or cannot tolerate
antidepressant treatments.
Electroconvulsive therapy
(ECT) is a reasonable treatment option for treatment-resistant depression
(TRD). With ECT, electricity is delivered to the cortex through scalp
electrodes applied over frontal and/or temporal cortical regions. A generalized
cortical seizure is produced, and the patient is kept paralyzed and unconscious
during the treatment to prevent harm from a tonic-clonic seizure. Response
rates with ECT, even in treatment-resistant patients, can be as high as 50% to
90%.2-5 ECT also reduces suicide attempt rates and has been shown to
reduce overall mortality rates in depressed patients over a 3-year follow-up.1,3
Despite its potential benefits, ECT still has significant risks and side
effects including post-ictal confusion, transient memory disturbance, and
cardiopulmonary complications.6-8 These risks, as well as societal
stigmatization, have significantly limited the use of ECT, and many patients
(and often their healthcare providers) are reluctant to accept this treatment.
Given the efficacy of ECT, which provides generalized
electrical stimulation to the brain, there is a growing interest in identifying
techniques for focally stimulating the brain. Focal brain stimulation might
possibly achieve antidepressant efficacy equivalent, or nearly equivalent, to
that seen with ECT, without the associated cognitive and anesthesia risks.
Transcranial magnetic stimulation (TMS), vagus-nerve
stimulation (VNS), and deep-brain stimulation (DBS) are three established
techniques for achieving focal brain stimulation. These techniques have been
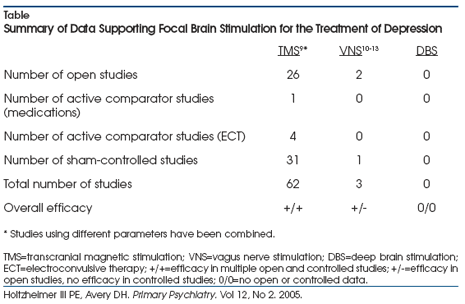
investigated as potential treatments for depression (Table).9-13
This article will describe these techniques and discuss the data supporting
their safety and efficacy in treating depression.
Transcranial Magnetic Stimulation
When TMS is administered,
an electrical current is passed through an electromagnetic coil placed on the
scalp. A brief, rapidly changing magnetic field is created, inducing a small
electrical current within the underlying cortex. The area of stimulation is
relatively focal, and covers about a 2–4 cm diameter region of cortex when
using a “figure-of-eight�? coil; the region of stimulation is broader when using
a less focal circular coil.14 TMS effectively depolarizes cortical
neurons within the area of stimulation. For example, a TMS pulse applied over
the motor cortex results in contraction of the contralateral muscle.
Single-pulse TMS is established as a diagnostic and research tool in humans.15
Repetitive TMS (rTMS), in which repeated electrical pulses
are generated in the cortex, is a more recent development of TMS technology,
and is associated with unique neurophysiologic effects. For example, high
frequency (“fast�?) rTMS, defined as >1 Hz stimulation, has been shown to
cause an increase in motor cortical excitability that persists beyond the end of
stimulation.16 Conversely, low frequency (“slow�?) rTMS, defined as £1 Hz stimulation, has been shown to decrease
motor cortical excitability.17 rTMS has been used to map brain
functions such as speech, vision, movement, and attention, and has been
extensively studied as a potential treatment for depression. There is no need
for anesthesia when giving non-convulsive rTMS, and it can be given on an
outpatient basis.
In interpreting rTMS
studies in depression, it is important to recognize the variability in
treatment parameters possible with rTMS, and to recognize that different
parameters and parameter combinations may have differential neurophysiological
effects. For example, stimulation frequency can be either slow or fast, with
varying effects on motor cortical excitability as described above. However,
fast rTMS can be given at different frequencies (eg, 5 Hz, 10 Hz, or 20 Hz),
and there is some evidence that different types of “fast�? rTMS (eg, 10 Hz
versus 20 Hz) may exert different physiologic effects.18
Stimulation intensity can
also be varied. Typically, intensity is expressed as a percentage of the
subject’s motor threshold (MT) for the abductor pollicis brevis or first dorsal
interosseous muscle. Some rTMS studies have used subthreshold stimulation (eg,
80% MT), while others have used threshold or suprathreshold stimulation (100%
to 110% MT).
In addition to stimulation frequency and intensity, rTMS
parameters can vary by train duration (length of a “burst�? of pulses at a given
frequency), number of trains per session, number of treatment sessions,
intertrain interval, and stimulation location (eg, which part of the cortex is
targeted). The neurophysiologic effects of rTMS most likely represent an
interaction of all of these parameters; for example, the risk that rTMS will
induce a generalized seizure is at least partially determined by an interaction
of intensity with frequency and train duration.19
Given this potential
variability in rTMS parameters, it is perhaps not surprising that treatment
studies of rTMS in depression have varied considerably in parameter selection.
This may in part explain the variability in treatment response between studies
(discussed below), although patient factors likely play a role as well. The
data supporting rTMS as a treatment for depression are reviewed below, and the
factors potentially affecting treatment response are discussed in more detail.
Open and Early Controlled Studies
Hoflich and colleagues20
first reported modest antidepressant effects of rTMS in one of two patients.
Since that time, several open and controlled studies have been conducted. The
earliest of these stimulated various cortical sites (eg, vertex and motor
cortex) using very slow rTMS (0.02–0.5 Hz) at intensities 80% to 130% MT for
five or fewer sessions. These studies generally showed very modest improvements
in depression ratings (see Burt and colleagues21 for a review). Most
subsequent studies applied rTMS to the left dorsolateral prefrontal cortex
(DLPFC), based on evidence that this region appeared to be functionally
involved in mood regulation and depression.22,23 These studies also
used fast rTMS and showed more substantial improvements in depression.24-26
For example, Epstein and colleagues27 found a 56% response rate in
32 medication-resistant patients treated with five sessions of 10 Hz, left
DLPFC rTMS at 110% MT intensity. Additionally, open studies of slow (0.5–1 Hz)
rTMS applied to the right DLPFC found mean decreases in Hamilton Rating Scale
for Depression scores of 31% to 42%.28,29
While these early studies did not definitively demonstrate
antidepressant effects for rTMS, they did help define which rTMS parameters
were likely to be therapeutic. It appeared from these data that high-frequency
rTMS applied to the left DLPFC likely resulted in antidepressant effects; it
was also suggested that low-frequency rTMS applied to the right DLPFC might be
effective as well.
High-Frequency, Left DLPFC rTMS
The vast majority of
sham-controlled studies of rTMS in TRD have investigated high-frequency (5–20
Hz) rTMS applied to the left DLPFC. These studies have used a range of
intensities (from sub- to supra-threshold) and various treatment durations
(5–20 treatment sessions). A number of meta-analyses have evaluated these
studies, each analysis using somewhat different inclusion/exclusion criteria
and statistical methods. These analyses agreed that high-frequency (³5 Hz), left DLPFC rTMS given for at least 10 daily
sessions results in statistically significant antidepressant effects.21,30-32
Kozel and colleagues31 also demonstrated that, while publication
bias for rTMS studies likely existed, 20–55 similarly-sized negative studies
would be needed to make the mean effect size for rTMS statistically
nonsignificant. Given that published and ongoing rTMS depression studies
throughout the world are tracked by the International Society for Transcranial
Stimulation,9 it is extremely unlikely that this many negative
studies of rTMS have been conducted but not reported. Therefore, it is
reasonable to conclude that high-frequency, left DLPFC rTMS, given for at least
10 sessions, does indeed have statistically significant antidepressant effects.
Despite this, it remains
unclear whether high-frequency, left DLPFC rTMS can produce clinically
meaningful effects. Every meta-analysis of rTMS has cautioned that, while rTMS
may have statistically significant effects, the degree of antidepressant
response in these studies is modest at best.20,30-32 Indeed,
Holtzheimer and colleagues30 showed that the average response rate
across all studies using high-frequency, left DLPFC rTMS was 18% for active
rTMS and 2% for sham rTMS. While this difference in response rates between
active and sham rTMS is of similar magnitude as for many antidepressant
studies, the response rate for actively-treated patients is still quite low.
One explanation for this
low response rate is simply that rTMS does not produce clinically significant
antidepressant effects. However, the response rate across studies varies
widely, and most studies of rTMS have described some subjects with rather
dramatic responses to rTMS treatment. Therefore, another explanation for the
low response rate across studies is that patient and treatment factors can
significantly influence response to rTMS.
Patient factors that influence response to rTMS likely
include degree of treatment resistance, duration of current depressive episode,
presence of psychosis, and age. Patients with TRD generally have low response
rates to all antidepressant treatments, including ECT.5,33
Therefore, treatment response expectations for rTMS should be lower when
studying treatment-resistant patients. Duration of current depressive episode
may also decrease likelihood of antidepressant response5,34,35;
patients with depressive episodes longer than 4 years may be unlikely to
respond to rTMS.34 As well, patients with psychotic features may not
respond well to rTMS treatment,36 and older patients may not respond
as well to rTMS as younger patients.37,38
Treatment factors which
may influence antidepressant response to high-frequency, left DLFPC rTMS likely
include number of treatment sessions and stimulation intensity. The majority of
studies of rTMS in depression have treated with 10 or fewer daily sessions.
Additionally, many of these studies have used subthreshold stimulation
intensity. One analysis of the rTMS literature found that studies using more
treatment sessions (>10) and higher treatment intensities (>100% MT)
demonstrated greater antidepressant response rates.39 More recent
studies also suggest that subthreshold intensity rTMS may not be as effective
in treating depression.40,41 A recently completed sham-controlled
study of rTMS in our center investigated 15 sessions of 10 Hz, 110% MT, left
DLPFC rTMS in 68 patients with TRD.42 Response rates were 31% for
active rTMS and 6% for sham rTMS; remission rates were 20% for active rTMS and
3% for sham rTMS. Therefore, by treating with more than 10 treatment sessions
at suprathreshold stimulation intensities, it may be possible to increase the
antidepressant response rate to rTMS.
Additional evidence for
the clinical efficacy of high-frequency, left DLPFC rTMS comes from comparisons
of rTMS with ECT. Four open-label studies have directly compared rTMS to ECT in
treatment-resistant depressed patients.36,43-45 While one of these
studies showed ECT to be superior to rTMS in treating patients with psychotic
depression,36 these studies generally showed no difference in
antidepressant response between rTMS and ECT in nonpsychotic depressed
patients. Of note, all of these studies used more than 10 treatment sessions.
While these studies were not powered to demonstrate statistical equivalence
between the two treatments, they are still suggestive that rTMS can have
antidepressant effects at least similar to ECT in some patients. However,
important caveats in interpreting these studies are that there was no sham
control and that ECT parameters were not consistently optimized (although ECT
response rates were quite similar to those often seen in treatment-resistant
patients).
In summary, current data
support the conclusion that high-frequency, left DLPFC rTMS produces
statistically significant antidepressant effects. While the clinical
significance of these effects has not yet been conclusively demonstrated,
studies comparing rTMS to ECT and a recent sham-controlled study using higher
intensity stimulation over 15 sessions suggest that high-frequency, left DLPFC
rTMS may indeed have clinically relevant antidepressant effects. Larger, more
definitive trials are needed to confirm this.
Low-Frequency, Right DLPFC rTMS
A small number of open studies suggest low-frequency, right
DLPFC rTMS may also have antidepressant effects.28,29,46 Three
sham-controlled studies have also shown statistically significant
antidepressant effects for low-frequency, right-sided rTMS. Klein and
colleagues47 treated 67 patients with 10 sessions of 1 Hz, right
DLPFC rTMS and found a 49% response rate in the active rTMS group and a 25%
response rate in the sham rTMS group. Notably, inclusion in this study was not
limited to TRD Patients, perhaps explaining the relatively larger sham and rTMS
response rates seen. Fitzgerald and colleagues48 compared 10 Hz,
left DLPFC rTMS to 1 Hz, right DLPFC rTMS to sham rTMS in 60 patients with TRD;
all patients received 10 sessions of double-blind treatment. Both active rTMS
conditions were superior to sham stimulation, and there was no significant
difference between the two active groups. Kaufmann and colleagues49
randomized 12 patients with TRD to receive 10 sessions of active or sham 1 Hz
rTMS to the right DLPFC. Four of seven patients (57%) in the active rTMS group
met both response and remission criteria. Two of five patients (40%) in the
sham group met response criteria and one of five patients (20%) met remission
criteria. Active rTMS patients showed a statistically significant reduction in
depression ratings from baseline to the final treatment session; sham-treated
patients showed no significant change in depression ratings.
In summary, a limited
database supports antidepressant efficacy for low-frequency (1 Hz), right DLPFC
rTMS in TRD. More research is needed to determine whether this form of rTMS
truly has statistically and clinically significant antidepressant effects.
Additionally, it will be important to determine whether certain patients are
more likely to respond to specific forms of rTMS.
Low-Frequency, Left DLPFC rTMS
A few small studies have
investigated low-frequency, left DLPFC stimulation. Padberg and colleagues50
compared five sessions of low-frequency, left DLPFC rTMS with high-frequency,
left DLPFC rTMS and sham rTMS. In this study, modest but statistically
significant antidepressant effects were found with low-frequency, left DLPFC
rTMS but not with high-frequency, left DLPFC or sham rTMS; shorter treatment
duration (only five sessions) may have confounded these results. Kimbrell and
colleagues51 found statistically significant antidepressant effects
with 10 sessions of 80% MT 1 Hz, left DLPFC rTMS, but not with 20 Hz, left
DLPFC or sham rTMS. Of note, this study used subthreshold stimulation intensity
which (as discussed above) may be less effective than higher stimulation
intensities. Rosenberg and colleagues52 randomized 15 patients with
posttraumatic stress disorder and major depression to receive 1 Hz or 5 Hz left
DLPFC rTMS. Response rates in the 1 Hz and 5 Hz groups were 67% and 83%
respectively, and there was no statistically significant difference between the
two conditions. However, the lack of a sham control group in this study limits
the conclusions that can be drawn. In summary, the database for low-frequency,
left DLPFC rTMS is suggestive of antidepressant effects, but is inconclusive.
Other rTMS Treatment Parameters
Several investigators have
explored the effects of various rTMS parameter combinations in patients with
depression. Loo and colleagues53 randomized 19 depressed patients to
receive sham stimulation or high-frequency (15 Hz) rTMS applied to both the
right and left DLPFC. All patients improved, but there was no statistical
difference between active and sham treated patients. Hausmann and colleagues54
randomized 38 patients to receive (1) high-frequency, left DLPFC rTMS; (2) a
combination of high-frequency left and low-frequency right DLPFC rTMS; or (3)
sham rTMS. At the beginning of the study, all patients were also started on
antidepressant medications. There was no significant difference in
antidepressant response between the three groups, but these results were
confounded by the “add-on�? nature of the trial and the use of heterogeneous
medication regimens. Conca and colleagues55 compared the antidepressant
effects of (1) high-frequency, left DLPFC rTMS plus low-frequency, right DLPFC
rTMS; (2) high-frequency, left DLPFC rTMS plus low-frequency, left DLPFC rTMS;
and (3) high-frequency, left DLPFC rTMS alone. No significant difference in
antidepressant response was seen between the groups.
A few investigators have
also explored using TMS to induce generalized seizures as a potential treatment
for depression (similar to ECT)—a technique called magnetic seizure therapy
(MST). While no definitive results have been reported, there is some evidence
that MST results in antidepressant effects with fewer cognitive side effects
than ECT.56,57 It is unclear whether MST is more or less efficacious
than nonconvulsive TMS.
These studies highlight
the variability in TMS parameters. They also suggest that other treatment
parameters may have antidepressant effects. However, the data are too sparse to
draw firm conclusions regarding the relative efficacy of TMS given these
parameters.
rTMS Side Effects and Safety
Studies of rTMS have shown it to be safe and well-tolerated.30
Despite extensive neuropsychological testing, rTMS has consistently proven to
have no negative cognitive side effects.58,59 While transient
auditory threshold increases have been reported with rTMS,58 this
can be eliminated with the use of foam earplugs. Some patients report a mild
headache during and/or following rTMS, but this is typically short-lived and
easily treated with over-the-counter analgesics.
The most concerning risk
of rTMS is a generalized seizure. Several reports have shown that high
frequency rTMS can induce a generalized seizure in epileptic and nonepileptic
individuals.19 Low-frequency rTMS does not appear to increase the
risk of seizure.19 Factors which increase the risk of seizure with
rTMS include other risk factors for seizure in the patient (eg, history of
epilepsy, proconvulsant medications), increased rTMS intensity, higher
stimulation frequencies, and short intertrain intervals.19 Safety
guidelines have been published to help investigators better control the risk of
seizure with rTMS.19 No seizure has been reported with rTMS used
according to these guidelines.
Use of rTMS in Special Populations
Geriatric Patients
One open study of rTMS in depressed patients found that older
patients had a much lower response rate than younger patients.38 A
double-blind, sham-controlled study38 in elderly patients with TRD
also failed to show statistically significant antidepressant effects for rTMS.
Interestingly, despite the absence of antidepressant effects in this study,
active rTMS was associated with statistically significant improvements in
cognitive function.60 A more recent study of rTMS for post-stroke
depression (mean patient age=64.8 years) demonstrated statistically significant
antidepressant effects for rTMS.61 One explanation for the mixed
results for rTMS in older persons may be greater prefrontal atrophy in older
age. Since the induced current from rTMS drops off rapidly with increasing
distance from the TMS coil,62 patients with prefrontal atrophy may
need higher stimulation intensities to achieve the same degree of cortical
stimulation as patients without atrophy. One open study in older patients with
depression showed that greater scalp-cortical distance at the prefrontal cortex
relative to the motor cortex (rather than absolute scalp-cortical distance at
the prefrontal cortex) negatively correlated with antidepressant response.63
Bipolar Depression
A number of studies of
rTMS for depression have included patients with bipolar depression. While many
of these patients have shown clinical improvements with rTMS, the numbers of
patients in each study were too small to draw definitive conclusions about the
relative efficacy and safety of rTMS in bipolar depression. Two small, sham-controlled
studies have specifically investigated high-frequency, left DLPFC rTMS in
patients with bipolar depression. One showed a modest, but statistically
significant benefit for rTMS over sham.64 The other showed no
statistically significant difference in antidepressant response between the
active and sham rTMS groups.65 Interestingly, several investigators
have reported the development of mania in patients receiving rTMS (some without
a prior diagnosis of bipolar disorder).66-69 More research is clearly
needed to identify whether rTMS is a safe and efficacious treatment for bipolar
depression.
Children and Adolescents
To date there are no
published trials of rTMS in children or adolescents. Walter and colleagues70
collated all known cases of rTMS use in children and adolescents and identified
only one center that had treated seven younger patients with a variety of
diagnoses (three with unipolar depression, one with bipolar depression, and
three with schizophrenia). Clinical improvement was seen in all patients except
one with unipolar depression and one with bipolar depression. To date, the
safety and efficacy of rTMS in younger persons is unknown.
Pregnancy
Nahas and colleagues71
reported a single case of a depressed pregnant woman who was successfully
treated during her second trimester with rTMS. No apparent adverse effects for
the patient or fetus were identified. Still, the safety of TMS and rTMS during
pregnancy has not yet been established.
Review of rTMS Studies: Limitations
An important limitation of all rTMS studies to date is small
sample size. Only two studies of rTMS in depression have included more than 30
patients per treatment arm.42,47 Additionally, the clinical
significance of rTMS has yet to be proven. Heterogeneity of patient and treatment
parameters in the rTMS literature limit firm conclusions as to which patients
will best respond to which types of treatment. Larger trials using optimized
rTMS are needed to better clarify these issues.
In relation, the optimal
parameters for rTMS as a treatment for depression are unknown. While active
rTMS appears to have greater antidepressant properties than sham rTMS, the
overall antidepressant response rate to rTMS is relatively low compared to
other antidepressant treatments. However, high-frequency, left DLPFC rTMS is
the only type of rTMS that has been extensively studied, and the majority of
these studies have used 10 or fewer treatment sessions and treatment
intensities at or below motor threshold. For high-frequency, left DLPFC rTMS,
it appears that higher intensities, longer treatment courses, and greater total
number of pulses may be more effective.39 It is possible that other
types of rTMS (eg, low-frequency, right DLPFC or combination left-right rTMS)
may be more effective for some patients. More research is needed to further
optimize treatment parameters for rTMS.
Another limitation of rTMS
treatment studies to date is the difficulty in finding a completely adequate
sham condition. Sham TMS is often given by rotating the coil away from the
scalp at either 45 or 90 degrees such that the focus of stimulation is away
from the cortex. However, such sham stimulation induces less of a scalp
sensation than active TMS. As well, sham stimulation at 45 degrees may induce
some activity in the cortex and may therefore be partially active.72
The mechanism of action of
rTMS is largely unknown. Preclinical studies suggest rTMS may induce
physiological and behavioral effects in animals similar to those elicited with
established antidepressant treatments.73,74 Imaging data also
suggest rTMS alters functioning of cortical and subcortical brain regions
implicated in the pathophysiology of depression.75,76 Still, an
important limitation of TMS is that it can only directly stimulate cortical
regions close to the coil—deeper brain structures involved in the
pathophysiology of depression cannot be directly targeted.
Summary
Multiple studies have investigated TMS as a treatment for
TRD. The strongest database supports antidepressant effects for high-frequency,
left DLPFC rTMS. Further, there is evidence that optimization of patient
factors and treatment parameters may improve response rates to this type of
rTMS. A smaller, but compelling database supports the antidepressant efficacy
of low-frequency, right DLPFC rTMS. Other studies suggest antidepressant
effects with other combinations of rTMS parameters, but the available data are
quite limited. If proven efficacious, rTMS would provide an important treatment
option for patients with TRD. Given that it is associated with relatively few
side effects and can be given on an outpatient basis, rTMS might provide an
alternative to some patients who might otherwise be referred for
electroconvulsive therapy (ECT). In sum, more research is needed to better
clarify the antidepressant effects of rTMS and to better identify which patient
and treatment factors are most predictive of antidepressant response.
Vagus-Nerve Stimulation
In VNS administration, an
electrical stimulator is connected to a patient’s left vagus nerve and attached
to a programmable pulse generator implanted subcutaneously in the patient’s
chest. The stimulator can be programmed to deliver electrical pulses to the
nerve at various frequencies and currents. Typically, stimulation parameters
are 0.25 milliampules, 20–30 Hz, 250–500 microsecond pulse width, and an on/off
cycle of 30 seconds on every 3–5 minutes. VNS is an established treatment for
treatment-resistant epilepsy.10 Based on mood improvements reported
by epileptic patients receiving VNS, it has also been suggested as a treatment
for depression.11 This idea is further supported by data that many
anticonvulsant agents can have antidepressant effects,77 and by
evidence that the anticonvulsant effects of ECT may be associated with its
antidepressant effects.78
One open study and one double-blind study have shown
antidepressant efficacy for VNS in epilepsy patients with comorbid depression.79,80
A single open study investigated VNS in 60 non-epileptic patients with profound
TRD, many of whom had previously failed ECT.12 One patient improved
during the 2-week, single-blind, no treatment recovery period following
implantation surgery. Of the remaining 59 patients, response and remission
rates after 10 weeks of treatment were 31% and 15% respectively. These results
suggested VNS might have clinically meaningful antidepressant effects in
treatment-resistant patients. However, a large, sham-controlled study failed to
show a statistically significant difference between sham and active VNS.11
Despite the negative findings of the sham-controlled study,
there is evidence that VNS may still be a useful treatment for some patients
with TRD. First, for the 59 patients enrolled in the open study of VNS,
response and remission rates increased to 45% and 27% at 1 year postsurgery,
and were 44% and 22% at 2 years postsurgery.13 Second, 1-year
response and remission rates for VNS-treated patients enrolled in the
sham-controlled study were 30% and 17% respectively.81 The 1-year
response and remission rates in a large group of demographically-comparable,
treatment-resistant depressed patients were 13% and 7% respectively.81
The 1-year response rate was significantly greater in the VNS group, and the
greater remission rate trended towards significance.
Side effects of VNS
surgery and treatment are generally mild.82 Surgical complications
are rare, with incision pain being the most commonly reported adverse event.
Common side effects of treatment include voice hoarseness and coughing. In
general, VNS appears to be well-tolerated and acceptable to patients.
The potential mechanism of action of VNS in treating epilepsy
and depression is currently unknown. Imaging studies suggest that VNS alters
functioning of cortical and subcortical brain regions known to be involved in
mood regulation and depression.83 However, as with TMS, VNS cannot
directly modulate the functioning of many brain regions implicated in the
pathophysiology of depression.
In summary, VNS is a relatively safe, well-tolerated
treatment that may have efficacy in TRD. Current efficacy data are mixed, with
a large, sham-controlled study finding no statistically significant acute
antidepressant effects. However, long-term follow-up data suggest response and
remission may increase over time in VNS-treated patients, and that these
increases may be statistically and perhaps clinically significant compared to
treatment-as-usual. More research is needed to clarify what role VNS may play
in the treatment of TRD.
Deep-Brain Stimulation
With DBS, a small electrical stimulator is implanted into a
defined brain location, often a subcortical area, and connected to a
programmable subcutaneous pulse generator. Bilateral DBS of the subthalamus or
globus pallidus is a relatively new, but generally accepted treatment for
treatment-resistant Parkinson’s disease.84
To date, there are no
published reports of DBS for TRD, although at least one study is currently
underway.85 Several lines of evidence support the investigation of
DBS as a treatment for depression. First, DBS has been clearly associated with
significant mood changes in patients with Parkinson’s disease86,87;
thus, direct modulation of mood with DBS is possible. Second, functional
imaging studies clearly implicate certain deep brain regions in the
pathophysiology of depression, such as the amygdala and anterior cingulate
cortex.76,88 Function in these regions could be directly modulated
with DBS; as discussed above, this is not possible with either TMS or VNS.
Third, two small case series have reported potential benefit from bilateral internal
capsule DBS in patients with severe, treatment-resistant obsessive-compulsive
disorder.85,89
Side effects of DBS
surgery and treatment can include intracranial hemorrhage, delirium, mood
changes (including depression and mania), and movement disorders.90
It is important to recognize that these effects were seen in patients with
Parkinson’s disease treated with DBS. It is not yet clear what side effects may
occur in non-Parkinsonian patients that undergo DBS surgery and long-term
treatment.
In summary, DBS is an
innovative technique for directly altering the function of deep brain
structures that has shown efficacy in treating treatment-resistant Parkinson’s
disease. While DBS has not yet shown efficacy in depressed patients, such
investigations are warranted.
Conclusion
Over the last several
years, focal brain stimulation techniques have been increasingly investigated
as potential treatments for TRD. The largest and most convincing database
supports antidepressant efficacy for high-frequency, left DLPFC rTMS.
A smaller database supports efficacy for low-frequency, right DLPFC rTMS. A
more mixed database supports the use of other combinations of rTMS parameters
in treating depression. While the clinical significance of rTMS has yet to be
established, the data suggest that careful attention to relevant patient and
treatment factors may improve antidepressant response rates. It is currently
unclear whether VNS has statistically or clinically significant antidepressant
effects, but long-term follow-up data with treatment-resistant patients suggest
this technique may be a useful treatment. DBS has not yet been studied as a
treatment for TRD, but such investigations appear warranted. Taken together,
these techniques offer an exciting new approach to the treatment of TRD, and
may improve
our understanding of the basic neurobiology of depression and antidepressant
treatments. PP
References
1. Avery D, Winokur G. Mortality in depressed patients
treated with electroconvulsive therapy and antidepressants. Arch Gen Psychiatry.
1976;33(9):1029-1037.
2. Avery D, Winokur G. Suicide, attempted suicide, and
relapse rates in depression. Arch
Gen Psychiatry. 1978;35(6):749-753.
3. Avery D, Winokur
G. The efficacy of electroconvulsive therapy and antidepressants in depression. Biol
Psychiatry. 1977;12(4):507-523.
4. Avery D, Lubrano
A. Depression treated with imipramine and ECT: the DeCarolis study
reconsidered. Am J Psychiatry. 1979;136(4B):559-562.
5. Prudic J, Haskett
RF, Mulsant B, et al. Resistance to antidepressant medications and short-term
clinical response to ECT. Am J Psychiatry. 1996;153(8):985-992.
6. Fink M. Convulsive
therapy: a review of the first 55 years. J Affect Disord. 2001;63(1-3):1-15.
7. Rose D, Fleischmann P, Wykes T, Leese M, Bindman J.
Patients’ perspectives on electroconvulsive therapy: systematic review. BMJ. 2003;326(7403):1363.
8. Zielinski RJ, Roose SP, Devanand DP, Woodring S,
Sackeim HA. Cardiovascular complications of ECT in depressed patients with
cardiac disease. Am J
Psychiatry. 1993;150(6):904-909.
9. Avery D, George M, Holtzheimer P. The
Avery-George-Holtzheimer Database of Studies of rTMS in Depression.
International Society for Transcranial Stimulation. Available at:
http://www.ists.unibe.ch. Accessed: November 2004.
10. Ben-Menachem E. Vagus-nerve stimulation for the
treatment of epilepsy. Lancet
Neurol. 2002;1(8):477-482.
11. George MS, Rush AJ, Sackeim HA, Marangell LB. Vagus
nerve stimulation (VNS): utility in neuropsychiatric disorders. Int J Neuropsychopharmacol.
2003;6(1):73-83.
12. Sackeim HA, Rush AJ, George MS, et al. Vagus nerve
stimulation (VNS) for treatment-resistant depression: efficacy, side effects,
and predictors of outcome. Neuropsychopharmacology.
2001;25(5):713-728.
13. Rush AJ, George MS, Sackeim HA, al. e. Continuing
benefit of VNS therapy over 2 years for treatment-resistant depression. Paper
presented at: 41st American College of Neuropsychopharmacology Annual Meeting;
December 8-12, 2002; San Juan, Puerto Rico.
14. Pascual-Leone A, Davey N, Rothwell J, Wassermann E,
Puri B. Handbook of
Transcranial Magnetic Stimulation. London, England: Arnold; 2002.
15. Anand S, Hotson J. Transcranial magnetic stimulation:
Neurophysiological applications and safety. Brain
Cogn. 2002;50(3):366-386.
16. Pascual-Leone A, Valls-Sole J, Wassermann EM, Hallett
M. Responses to rapid-rate transcranial magnetic stimulation of the human motor
cortex. Brain.
1994;117(Pt 4):847-858.
17. Chen R, Classen J, Gerloff C, et al. Depression of
motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. 1997;48(5):1398-1403.
18. Maeda F, Keenan JP, Tormos JM, Topka H, Pascual-Leone
A. Modulation of corticospinal excitability by repetitive transcranial magnetic
stimulation. Clin
Neurophysiol. 2000;111(5):800-805.
19. Wassermann EM. Risk and safety of repetitive
transcranial magnetic stimulation: report and suggested guidelines from the
International Workshop on the Safety of Repetitive Transcranial Magnetic
Stimulation, June 5-7, 1996. Electroencephalogr
Clin Neurophysiol. 1998;108(1):1-16.
20. Hoflich G, Kasper S, Hufnagel A, S R, HJ M. Application
of transcranial magnetic stimulation in treatment of drug-resistant major
depression-- A report of two cases. Hum
Psychopharmacol. 1993;8:361-365.
21. Burt T, Lisanby SH,
Sackeim HA. Neuropsychiatric applications of transcranial magnetic stimulation:
a meta-analysis. Int J Neuropsychopharmacol. 2002;5(1):73-103.
22. Bench CJ, Friston KJ, Brown RG, Scott LC, Frackowiak
RS, Dolan RJ. The anatomy of melancholia--focal abnormalities of cerebral blood
flow in major depression. Psychol
Med. 1992;22(3):607-615.
23. Biver F, Goldman S,
Delvenne V, et al. Frontal and parietal metabolic disturbances in unipolar
depression. Biol Psychiatry.
1994;36(6):381-388.
24. George MS, Wassermann EM, Williams WA, et al. Daily
repetitive transcranial magnetic stimulation (rTMS) improves mood in
depression. Neuroreport.
1995;6(14):1853-1856.
25. Pascual-Leone A, Rubio B, Pallardo F, Catala MD.
Rapid-rate transcranial magnetic stimulation of left dorsolateral prefrontal
cortex in drug-resistant depression. Lancet.
1996;348(9022):233-237.
26. Epstein C, Figiel G, McDonald W, Amazon-Leece J, Figiel
L. Rapid rate transcranial magnetic stimulation in young and middle-aged
refractory depressed patients. Psychiatr
Ann. 1998;28(1):36-39.
27. Epstein C, Figiel
G, McDonald W, Amazon-Leece J, Figiel L. Rapid rate transcranial magnetic
stimulation in young and middle-aged refractory depressed patients. Psych Ann. 1998;28(1):36-39.
28. Feinsod M, Kreinin
B, Chistyakov A, Klein E. Preliminary evidence for a beneficial effect of
low-frequency, repetitive transcranial magnetic stimulation in patients with
major depression and schizophrenia. Depress Anxiety. 1998;7(2):65-68.
29. Menkes DL, Bodnar P, Ballesteros RA, Swenson MR. Right
frontal lobe slow frequency repetitive transcranial magnetic stimulation (SF
r-TMS) is an effective treatment for depression: a case-control pilot study of
safety and efficacy. J Neurol
Neurosurg Psychiatry. 1999;67(1):113-115.
30. Holtzheimer PE, 3rd,
Russo J, Avery DH. A meta-analysis of repetitive transcranial magnetic
stimulation in the treatment of depression. Psychopharmacol Bull. 2001;35(4):149-169.
31. Kozel FA, George MS. Meta-analysis of left prefrontal
repetitive transcranial magnetic stimulation (rTMS) to treat depression. J Psychiatr Pract.
2002;8(5):270-275.
32. Martin JL, Barbanoj
MJ, Schlaepfer TE, Thompson E, Perez V, Kulisevsky J. Repetitive transcranial
magnetic stimulation for the treatment of depression: Systematic review and
meta-analysis. Br J Psychiatry. 2003;182:480-491.
33. Nierenberg AA, Feighner JP, Rudolph R, Cole JO,
Sullivan J. Venlafaxine for treatment-resistant unipolar depression. J Clin Psychopharmacol.
1994;14(6):419-423.
34. Holtzheimer PE, 3rd, Russo J, Claypoole KH, Roy-Byrne
P, Avery DH. Shorter duration of depressive episode may predict response to
repetitive transcranial magnetic stimulation. Depress
Anxiety. 2004;19(1):24-30.
35. Nelson JC, Mazure
CM, Jatlow PI. Characteristics of desipramine-refractory depression. J Clin
Psychiatry. 1994;55(1):12-19.
36. Grunhaus L, Dannon PN, Schreiber S, et al. Repetitive
transcranial magnetic stimulation is as effective as electroconvulsive therapy
in the treatment of nondelusional major depressive disorder: an open study. Biol Psychiatry. 2000;47(4):314-324.
37. Figiel GS, Epstein C, McDonald WM, et al. The use of
rapid-rate transcranial magnetic stimulation (rTMS) in refractory depressed
patients. J Neuropsychiatry
Clin Neurosci. 1998;10(1):20-25.
38. Manes F, Jorge R, Morcuende M, Yamada T, Paradiso S,
Robinson RG. A controlled study of repetitive transcranial magnetic stimulation
as a treatment of depression in the elderly. Int
Psychogeriatr. 2001;13(2):225-231.
39. Gershon AA, Dannon PN, Grunhaus L. Transcranial
magnetic stimulation in the treatment of depression. Am J Psychiatry. 2003;160(5):835-845.
40. Boutros NN, Gueorguieva R, Hoffman RE, Oren DA,
Feingold A, Berman RM. Lack of a therapeutic effect of a 2-week sub-threshold
transcranial magnetic stimulation course for treatment-resistant depression. Psychiatry Res. 2002;113(3):245-254.
41. Padberg F, Zwanzger
P, Keck ME, et al. Repetitive transcranial magnetic stimulation (rTMS) in major
depression: relation between efficacy and stimulation intensity. Neuropsychopharmacology.
2002;27(4):638-645.
42. Avery DH,
Holtzheimer PE, Fawaz W, et al. Repetitive transcranial magnetic stimulation
(rTMS) is clinically effective in treatment-resistant major depression. Paper
presented at: 59th Annual Meeting of the Society of Biological Psychiatry;
April 29-May 1, 2004; New York, NY.
43. Grunhaus L,
Schreiber S, Dolberg OT, Polak D, Dannon PN. A randomized controlled comparison
of electroconvulsive therapy and repetitive transcranial magnetic stimulation
in severe and resistant nonpsychotic major depression. Biol Psychiatry. 2003;53(4):324-331.
44. Janicak PG, Dowd SM, Martis B, et al. Repetitive
transcranial magnetic stimulation versus electroconvulsive therapy for major
depression: preliminary results of a randomized trial. Biol Psychiatry.
2002;51(8):659-667.
45. Pridmore S, Bruno R, Turnier-Shea Y, Reid P, Rybak M.
Comparison of unlimited numbers of rapid transcranial magnetic stimulation
(rTMS) and ECT treatment sessions in major depressive episode. Int J Neuropsychopharmacol.
2000;3(2):129-134.
46. Brasil-Neto JP, Boechat-Barros R, da Mota-Silveira DA.
[The use of slow-frequency transcranial magnetic stimulation in the treatment
of depression at Brasilia University Hospital: preliminary findings]. Arq Neuropsiquiatr. 2003;61(1):83-86.
Epub 2003 Apr 2016.
47. Klein E, Kreinin I,
Chistyakov A, et al. Therapeutic efficacy of right prefrontal slow repetitive
transcranial magnetic stimulation in major depression: a double-blind
controlled study. Arch Gen Psychiatry. 1999;56(4):315-320.
48. Fitzgerald PB, Brown TL, Marston NA, Daskalakis ZJ, De
Castella A, Kulkarni J. Transcranial magnetic stimulation in the treatment of
depression: a double-blind, placebo-controlled trial. Arch Gen Psychiatry. 2003;60(10):1002-1008.
49. Kauffmann CD, Cheema MA, Miller BE. Slow right
prefrontal transcranial magnetic stimulation as a treatment for
medication-resistant depression: a double-blind, placebo-controlled study. Depress Anxiety.
2004;19(1):59-62.
50. Padberg F, Zwanzger P, Thoma H, et al. Repetitive transcranial
magnetic stimulation (rTMS) in pharmacotherapy-refractory major depression:
comparative study of fast, slow and sham rTMS. Psychiatry Res. 1999;88(3):163-171.
51. Kimbrell TA, Little JT, Dunn RT, et al. Frequency
dependence of antidepressant response to left prefrontal repetitive
transcranial magnetic stimulation (rTMS) as a function of baseline cerebral
glucose metabolism. Biol
Psychiatry. 1999;46(12):1603-1613.
52. Rosenberg PB, Mehndiratta RB, Mehndiratta YP, Wamer A,
Rosse RB, Balish M. Repetitive transcranial magnetic stimulation treatment of
comorbid posttraumatic stress disorder and major depression. J Neuropsychiatry Clin Neurosci.
2002;14(3):270-276.
53. Loo CK, Mitchell PB, Croker VM, et al. Double-blind
controlled investigation of bilateral prefrontal transcranial magnetic
stimulation for the treatment of resistant major depression. Psychol Med.
2003;33(1):33-40.
54. Hausmann A, Kemmler G, Walpoth M, et al. No benefit
derived from repetitive transcranial magnetic stimulation in depression: a
prospective, single centre, randomised, double blind, sham controlled “add on“ trial. J Neurol Neurosurg
Psychiatry. 2004;75(2):320-322.
55. Conca A, Di Pauli J, Beraus W, et al. Combining high
and low frequencies in rTMS antidepressive treatment: preliminary results. Hum Psychopharmacol.
2002;17(7):353-356.
56. Lisanby SH, Luber B, Schlaepfer TE, Sackeim HA. Safety
and feasibility of magnetic seizure therapy (MST) in major depression:
randomized within-subject comparison with electroconvulsive therapy. Neuropsychopharmacology. 2003;28(10):1852-1865.
57. Kosel M, Frick C,
Lisanby SH, Fisch HU, Schlaepfer TE. Magnetic seizure therapy improves mood in
refractory major depression. Neuropsychopharmacology. 2003;28(11):2045-2048.
58. Loo C, Sachdev P, Elsayed H, et al. Effects of a 2- to
4-week course of repetitive transcranial magnetic stimulation (rTMS) on
neuropsychologic functioning, electroencephalogram, and auditory threshold in
depressed patients. Biol
Psychiatry. 2001;49(7):615-623.
59. Martis B, Alam D,
Dowd SM, et al. Neurocognitive effects of repetitive transcranial magnetic
stimulation in severe major depression. Clin Neurophysiol. 2003;114(6):1125-1132.
60. Moser DJ, Jorge RE, Manes F, Paradiso S, Benjamin ML,
Robinson RG. Improved executive functioning following repetitive transcranial
magnetic stimulation. Neurology.
2002;58(8):1288-1290.
61. Jorge RE, Robinson
RG, Tateno A, et al. Repetitive transcranial magnetic stimulation as treatment
of poststroke depression: a preliminary study. Biol Psychiatry. 2004;55(4):398-405.
62. Kozel FA, Nahas Z,
deBrux C, et al. How coil-cortex distance relates to age, motor threshold, and
antidepressant response to repetitive transcranial magnetic stimulation. J Neuropsychiatry
Clin Neurosci.
2000;12(3):376-384.
63. Mosimann UP, Marre SC, Werlen S, et al. Antidepressant effects of repetitive transcranial magnetic
stimulation in the elderly: correlation between effect size and coil-cortex
distance. Arch Gen Psychiatry.
2002;59(6):560-561.
64. Dolberg OT, Dannon
PN, Schreiber S, Grunhaus L. Transcranial magnetic stimulation in patients with
bipolar depression: a double blind, controlled study. Bipolar Disord. 2002;4(Suppl 1):94-95.
65. Nahas Z, Kozel FA,
Li X, Anderson B, George MS. Left prefrontal transcranial magnetic stimulation
(TMS) treatment of depression in bipolar affective disorder: a pilot study of
acute safety and efficacy. Bipolar Disord. 2003;5(1):40-47.
66. Garcia-Toro M. Acute manic symptomatology during
repetitive transcranial magnetic stimulation in a patient with bipolar
depression. Br J Psychiatry. 1999;175:491.
67. Dolberg OT, Schreiber S, Grunhaus L. Transcranial
magnetic stimulation-induced switch into mania: a report of two cases. Biol Psychiatry.
2001;49(5):468-470.
68. Sakkas P, Mihalopoulou P, Mourtzouhou P, et al.
Induction of mania by rTMS: report of two cases. Eur Psychiatry. 2003;18(4):196-198.
69. Ella R, Zwanzger P, Stampfer R, et al. Switch to mania
after slow rTMS of the right prefrontal cortex. J Clin Psychiatry. 2002;63(3):249.
70. Walter G, Tormos JM, Israel JA, Pascual-Leone A.
Transcranial magnetic stimulation in young persons: a review of known cases. J Child Adolesc Psychopharmacol.
2001;11(1):69-75.
71. Nahas Z, Bohning DE, Molloy MA, Oustz JA, Risch SC, George MS. Safety and feasibility of repetitive transcranial magnetic stimulation
in the treatment of anxious depression in pregnancy: a case report. J Clin Psychiatry.
1999;60(1):50-52.
72. Lisanby SH, Gutman D, Luber B, Schroeder C, Sackeim HA.
Sham TMS: intracerebral measurement of the induced electrical field and the
induction of motor-evoked potentials. Biol
Psychiatry. 2001;49(5):460-463.
73. Post A, Keck ME.
Transcranial magnetic stimulation as a therapeutic tool in psychiatry: what do
we know about the neurobiological mechanisms? J Psychiatr Res. 2001;35(4):193-215.
74. Muller MB, Toschi
N, Kresse AE, Post A, Keck ME. Long-term repetitive transcranial magnetic
stimulation increases the expression of brain-derived neurotrophic factor and
cholecystokinin mRNA, but not neuropeptide tyrosine mRNA in specific areas of
rat brain. Neuropsychopharmacology.
2000;23(2):205-215.
75. Speer AM, Kimbrell
TA, Wassermann EM, et al. Opposite effects of high and low frequency rTMS on
regional brain activity in depressed patients. Biol Psychiatry. 2000;48(12):1133-1141.
76. Mayberg HS. Modulating dysfunctional limbic-cortical
circuits in depression: towards development of brain-based algorithms for
diagnosis and optimised treatment. Br
Med Bull. 2003;65:193-207.
77. APA. Practice guideline for the treatment of patients
with bipolar disorder (revision). Am
J Psychiatry. Apr 2002;159(4 Suppl):1-50.
78. Sackeim HA. The anticonvulsant hypothesis of the
mechanisms of action of ECT: current status. J
ECT. 1999;15(1):5-26.
79. Harden CL, Pulver MC, Ravdin LD, Nikolov B, Halper JP,
Labar DR. A pilot study of mood in epilepsy patients treated with vagus nerve
stimulation. Epilepsy Behav.
2000;1(2):93-99.
80. Elger G, Hoppe C, Falkai P, Rush AJ, Elger CE. Vagus
nerve stimulation is associated with mood improvements in epilepsy patients. Epilepsy Res.
2000;42(2-3):203-210.
81. Dunner DL, Russell
JM. Long-term outcomes in pharmacoresistant depression with vagus nerve
stimulation (VNS) therapy. Paper presented at: 42nd Annual Meeting of the American College of Neuropsychopharmacology; December 7-11, 2003; San Juan, Puerto Rico.
82. Ben-Menachem E. Vagus nerve stimulation, side effects,
and long-term safety. J Clin
Neurophysiol. 2001;18(5):415-418.
83. Chae JH, Nahas Z, Lomarev M, et al. A review of
functional neuroimaging studies of vagus nerve stimulation (VNS). J Psychiatr Res. 2003;37(6):443-455.
84. Deuschl G,
Wenzelburger R, Kopper F, Volkmann J. Deep brain stimulation of the subthalamic
nucleus for Parkinson’s disease: a therapy approaching evidence-based standards. J
Neurol. 2003;250(Suppl 1):I43-46.
85. Greenberg B. Deep
brain stimulation: Clinical findings in OCD and depression. Paper presented at:
59th Annual Meeting of the Society of Biological Psychiatry; April 29-May 1,
2004; New York, NY.
86. Bejjani BP, Damier P, Arnulf I, et al. Transient acute
depression induced by high-frequency deep-brain stimulation. N Engl J Med.
1999;340(19):1476-1480.
87. Stefurak T, Mikulis D, Mayberg H, et al. Deep brain
stimulation for Parkinson‘s disease dissociates mood and motor circuits: a
functional MRI case study. Mov
Disord. 2003;18(12):1508-1516.
88. Drevets WC. Neuroimaging abnormalities in the amygdala
in mood disorders. Ann N Y
Acad Sci. 2003;985:420-444.
89. Nuttin BJ, Gabriels LA, Cosyns PR, et al. Long-term
electrical capsular stimulation in patients with obsessive-compulsive disorder.
Neurosurgery.
2003;52(6):1263-1272; discussion 1272-1264.
90. Herzog J, Volkmann J, Krack P, et al. Two-year
follow-up of subthalamic deep brain stimulation in Parkinson’s disease. Mov Disord. 2003;18(11):1332-1337.