Print Friendly
Clinical Predictors in Major Depressive Disorder
Madhukar H. Trivedi, MD
Benji
T. Kurian, MD
Bruce D. Grannemann, MA
Dr. Trivedi is the Lydia Bryant Test Professor in Psychiatric Research, professor of psychiatry, and director of the Mood Disorders Research Program, Dr. Kurian is a post-doctoral research fellow; and Mr. Grannemann is a faculty member in the Department of Psychiatry at the University of Texas Southwestern in Dallas.
Abstract
Remission is the goal for current depression treatment. However, the Sequenced Treatment Alternatives to Relieve Depression
study has shown that the majority of patients will fail to achieve remission with a first-line antidepressant agent.
Previous research has attempted to identify which depression treatments are preferred for whom by assessing baseline
predictors. Of predictors, sex, age, severity of illness, depressive subtype, and comorbidity have predicted treatment
response/nonresponse. However, these predictors have not always provided meaningful clinical correlation. Furthermore,
based on the results of recent research, it is clear that clinicians need predictive variables to identify “next-best”
preferred depression treatments for patients. This article defines these predictive variables as process predictors—that
is they include clinical features that appear during the treatment process and are associated with outcomes.
INTRODUCTION
Depressive disorders are one of the leading causes of disability-adjusted life years, worldwide.1 In
the United States, depressive disorders account for >31 billion dollars a
year in lost employee productivity.2 This debilitating illness will affect up to 16.2% of Americans by the
end of their lifetime.3
Antidepressant medications are the most common form of treatment for
adults suffering from major depressive disorder (MDD).4 Among antidepressants, selective serotonin reuptake
inhibitors (SSRIs) are the most commonly prescribed agents in the US.4 However, based on recent clinical
trials evaluated in “real-world” settings, first-step treatment with an SSRI is only 33% effective in achieving remission
of depressive symptoms.5 Furthermore, about one-third of patients with MDD will become treatment resistant
(ie, remain symptomatic despite multiple trials of antidepressant medications).6
Since a large number of patients fail to respond to antidepressants,
an emphasis has been placed on identifying predictors of treatment success. To date, most research on predicting treatment
response has focused on baseline predictors. This article includes not only a review of new research on baseline predictors
but will also examine a new type of predictor—process predictors—that include changes that occur early in treatment,
which may relate to final outcomes and can be used to tailor treatment to individual patients once treatment has been
initiated.
CURRENT FINDINGS: Baseline Predictors
Baseline predictors are based on information that is available to
clinicians at the initiation of treatment. Baseline predictors are important because patient features at the point
of treatment initiation could inform clinicians as to what treatments may be best for a specific patient or which treatments
may be more effective than other treatments. Although this type of predictor would be of the greatest use to clinicians
and the majority of research has pursued the identification of baseline predictors, the majority of findings examining
baseline predictors seldom prove useful in the clinical setting because of their modest to small effect size.7 Baseline
predictors are divided here into five different categories: sociodemographic factors, depressive illness features,
symptom factors, comorbidity factors, and a review of the burgeoning field of biological predictors.
Sociodemographic Predictors
Most research to date has focused on assessing whether baseline factors (ie, sociodemographic, illness
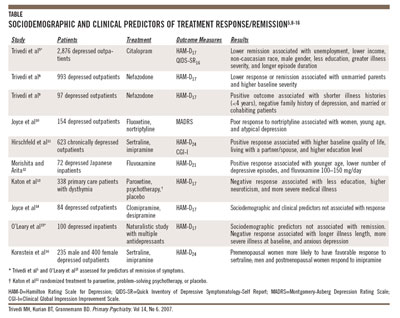
features, symptom presentation, and comorbid conditions) predict future antidepressant response (Table).5,8-16 Few
markers have been proven to reliably predict a positive antidepressant response.11,12,17-19 Some replicated
studies have forecasted that less education, single living status, and low baseline quality of life predict treatment
non-response to antidepressants.8,9,13,19,20 In fact, Trivedi and colleagues8,9 found that living
status (married or cohabiting) predicted better treatment response than living alone (single, engaged, divorced, widowed).
Furthermore, studies have reliably replicated the effect of gender and age; specifically, younger women are more likely
to respond to SSRIs and less likely to tolerate or respond to tricyclic antidepressants (TCAs).10,16,21 Additionally,
Kornstein and colleagues16 hypothesize that female sex hormones may account for this effect (ie, premenopausal
status may predict a better treatment response to SSRIs versus postmenopausal status).
Illness Features
Several illness characteristics can be used as negative predictors.
For example, patients with increased baseline depression severity are less likely to respond to antidepressants.5,15 Another
factor associated with MDD, the illness length both of the current episode5 and of prior episodes,15,22 has
shown to negatively predict treatment response. However, age of onset of first depressive episode has variably predicted
symptom response. Some studies find younger age of onset to have less favorable treatment response,15,23 while
others found no association.14,24 Thus, a lower rate of response with more severe illness, as indicated
by severity at baseline and longer duration of episodes.
Symptom Features
For years, clinical symptom presentations have been used to describe depressive subtypes. One of the
oldest subtypes is melancholic depression (ie, “endogenous depression”) which is described in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition,
Text Revision (DSM-IV-TR) as having an essential feature of “loss of interest or pleasure
in all, or almost all, activities or a lack of reactivity to usually pleasurable stimuli.”25 As with other
predictors of MDD, no clear factors associated with melancholia have reliably predicted treatment response to antidepressant
medications.26,27 Past studies have shown that £50% of patients suffering
from melancholic depression have abnormal baseline dexamethasone suppression tests; however, no correlation to pharmacologic
response has been associated.28,29
Another well-studied depressive subtype is atypical depression, which
is primarily characterized by mood reactivity or an ability to be “cheered up” by positive events.25,30 A
seminal finding by Quitkin and colleagues31 is that those suffering from atypical depression are more likely
to respond to monoamine oxidase inhibitors (MAOIs) than TCAs.31-33 However, when assessing SSRI versus MAOI
treatment response in depressed patients with atypical features, results have been mixed and unclear.10,34 With
regard to clinical prediction and further delineation of the atypical diagnoses, Stewart and colleagues35 found
that patients with atypical depression who had early onset of depressive illness and disease chronicity responded poorly
to TCAs.
Despite the fact that the DSM-IV-TR does not currently hold a specifier for depression with anxious features, a number of studies
have described this correlate in the literature.36-40 In fact, based on research from the Sequenced Treatment
Alternatives to Relieve Depression (STAR*D) trial, 46% of patients met baseline criteria for anxious depression.36 Literature
has shown that patients with MDD and comorbid anxiety are more likely to suffer increased disease burden and longer
duration of illness.39,41 Furthermore, some studies have shown that patients suffering from anxious depression
are less likely to respond to antidepressants,41-43 while other studies report similar response rates for
anxious and non-anxious depression, but a longer time to response for the former group.44,45
Irritability, although not a distinct depressive subtype, does correlate with increased depression severity
and further studies to assess the prediction of treatment response are warranted.46 Additionally, physical
pain is a common symptom accompanying MDD that appears to be associated with increased severity of illness and may have
a slightly better treatment response to dual-action antidepressants.47,48
Comorbidity Predictors
MDD that is comorbid with medical illnesses has also shown mixed results with regard to prediction, with
some large studies predicting a lower response rate to antidepressants, while other smaller studies report a potentially
insignificant difference between medical illness and no medical illness.49,50 Meanwhile, depressive illnesses
comorbid with personality disorders have been associated with both delayed response to treatment51 and a negative
predictive value.52,53
Neuroticism is a personality trait that is a reliable predictor of treatment non-response.13,18,54,55 Katon
and colleagues13 define patients with neuroticism as having low tolerance for stress, feeling alienated, having
low-self-esteem, and being anxious worriers, and feeling easily victimized and resentful.
Biological Predictors
This article seeks to provide clinicians with a practical guide to
predictors of treatment response and, although biological predictors (eg, neuroimaging, pharmacogenetics, quantitative
electroenchalography [QEEG]) are yet to be clinically applicable, it is important to present a brief introduction into
new directions in the field. A recent study reported that patients with hypofolatemia and/or associated brain white
matter hyperintensities, measured via magnetic resonance imaging (MRI), had a lower probability to respond to antidepressants.56
More recently, researchers have focused on pharmacogenetic predictors of antidepressant response, and
results have been mixed.57-59 The interplay of pharmacogenetic markers and pharmacologic response is complex
and potentially variable among ethnic populations.58,59 Researchers have also studied neurophysiologic factors
as potential predictors of treatment response, of which prefrontal QEEG cordance appears to hold the most promise.60-63 Perlis64 has
extensively described the role of genetic predictors.
Although the development of a clinical factor that reliably predicts
pharmacologic response is of great importance, a vast majority of patients will not achieve symptom remission with
a first-line agent. Thus, clinicians should devise a methodology to predict response for the “next-best treatment.”
The STAR*D study was designed and executed with the knowledge that depression is a chronic illness with the goal of
attaining remission in “real-world” settings.65 Therefore, it is necessary to identify process predictors
that will allow clinicians to identify next-best treatment options for patients who have failed prior approaches.
A critical factor in evaluating baseline predictor research is that the majority of research examines
one or two active treatment medications. Thus, identifying variables that could differentiate between treatments becomes
very difficult.
NEW DIRECTIONS: Process Predictors
Process predictors are based on information that becomes available
to the clinician during the process of treatment. Examples of process predictors include timing and nature of change
in symptom severity in the first few weeks of treatment, side-effect burden, and patient adherence. The concept of
process predictors has become important because of the new direction that depression treatment has taken over the last
10 years. Since the goal of treatment has become remission as opposed to response, results of large clinical trials
show that for the majority of patients, the first treatment is unlikely to achieve this goal.5 Thus, the
ability to predict early in the course of treatment which particular patients will achieve remission and which patients
will not achieve remission is vitally important.
A clinical example of a process predictor is using measures of depression
severity during the early weeks of treatment to predict antidepressant success/failure.66 That is, the changes
from baseline scores on standard symptom severity measures in the first few weeks of treatment could be used to predict
the patient’s final status. Other measures of this type may include treatment adherence, side-effect burden, or changes
in specific symptom clusters within the MDD domain, such as anxiety, cognition, and core emotions. Nierenberg and colleagues67 described
this approach in 1995, as they used changes from baseline in the first 4 weeks of treatment to predict final status
at the end of an 8-week trial. In this study, the authors found, for patients with a reduction in Hamilton Rating Scale
for Depression (HAM-D)68 scores of <20%, there was a <30% chance of showing a full response (response
defined as a 50% HAM-D improvement from baseline) on week 8.
Another study conducted by Nierenberg and colleagues69 was
designed to examine the timing of antidepressant response with fluoxetine. While there was no evidence for a specific
time for response, this study did show that patients who had not shown a response in the first 4 weeks of treatment
were unlikely to respond. In fact among patients who had not shown a response in the first 2 weeks of treatment, <75% showed response by the end of 8 weeks. This study suggests that the initial response to
treatment may be a critical predictor of whether or not a patient will eventually respond to a treatment.
In another study, Perlis and colleagues19 examined three
different augmentation strategies following a 4-week trial of fluoxetine 20 mg/day to assess predictors of treatment
augmentation. The researchers compared increasing the dose of fluoxetine, addition of desipramine, or addition of lithium.
While the study failed to find any predictors of specific treatment, the study reported three elements that predicted
whether or not patients would achieve a response. Two of these elements were baseline predictors marital status and
age of depression onset. In addition, the study also reported a process type predictor: the severity of patients’ HAM-D
score after 4 weeks of treatment predicted likelihood of response with the augmentation.
Trivedi and colleagues70 have also used changes in symptom status as a predictor of response.
In this study, patients who were enrolled in an open-label 12-week trial and had not shown a response by week 4 were
assessed. The changes in symptom clusters during the first 4 weeks of treatment were used to predict whether or not these
patients would achieve a full response by the end of the 12-week trial. Findings suggest that changes in the symptom
cluster scores between weeks 3 and 4 were best at determining which patients would eventually achieve a full response.
A discriminant analysis correctly identified 70% of the late responders and 64% of the non-responders.
In general, predictor research has focused on identifying variables
that inform clinicians of the best treatment for a given patient. However, emphasis on process predictor research is
designed to identify which patients should be continued on current treatment and which patients are unlikely to benefit
from the current treatment during various time points in the acute phase. This may be one of the most beneficial types
of prediction to reduce unnecessarily long and ineffective treatment trials, further enhancing the chances of identifying
the most effective treatment at the earliest time and potentially reducing attrition from treatment. This benefit is
particularly true if the treatment goal is to achieve full remission. Based on the STAR*D study, patients achieve only
modest rates of remission with the first treatment.5 Therefore, if researchers can shorten unsuccessful
treatment time, clinicians should be able to more rapidly switch patients to a more effective treatment.
DISCUSSION
Treatment of MDD is complicated because of available antidepressants’ slow onset and low remission rates;
uncertainty about the optimal duration of an adequate acute treatment trial; high rates of residual symptomatology; and
need for sequential treatment steps and associated response heterogeneity. Furthermore, the improvement trajectory after
an antidepressant is initiated as a determinant of eventual outcome has proved to be of limited utility. These difficulties
have led to a treatment process in clinical care that is based on trial and error until an effective treatment is identified.
The recently completed STAR*D trial found that up to 67% of patients can achieve remission with four treatment steps
used sequentially,5,71-76 and that well-defined measurement-based care can be used to tailor treatment during
each of the acute phase steps to maximize outcomes.5,77,78 (Measurement-based care involves use of response
as determined by rating scales to guide dose adjustment). One conclusion that can be drawn from the sequential treatment
trials is that there is a clear need for methods to predict which treatment will work for which patient. Well-defined
predictors are likely to assist in identification of the most appropriate treatment; determination of the duration of
an adequate treatment trial for a given patient; identification of the next best step treatment, and determination of
the nature and timing of intervention for residual or breakthrough symptoms during the long-term management of patients
with MDD. There are at least two types of clinical predictors that could be of use to practicing clinicians. First, information
used at the initiation of treatment can aid in the treatment choice. Second, information accrued during the treatment
course can determine whether continuation of the current treatment will produce full and sustained remission of depressive
symptoms.
Research on predictors has predominantly focused on identification of a number of variables that could
be used to predict outcomes for groups of patients as a whole. Thus, researchers focus on variables that help them understand
the underlying etiology or pathophysiology of the disorder. Since there are no direct or absolute measures of depression
(ie, the measurement of the underlying depression is typically based on patient reports or clinician observations of
patient behavior) there remains a fair amount of uncertainty about the contribution of each of the variables in predicting
outcomes. In contrast, clinicians have to apply the results of studies based on group data to individual patients. Specifically,
there is a need for predictors that can be used to guide treatment in terms of initiating the “right” antidepressant
treatment; determining the timing of a treatment change, and assessing the need for adjunctive treatments for associated
symptoms, side effects, or residual symptoms. Clinicians are also required to assist patients by educating them about
what to expect in terms of symptom change as well as disease self-management in order to personalize treatment and engage
the patient in the treatment process. Achieving these goals would not be a problem if the clinical and or biological
variables identified as significant predictors were more robust. However, in a typical depression study, any given predictor
is likely to explain only a small percentage of the variance. This results in a situation in which characteristics that
have been identified as predictors in research studies may not be of practical use in predicting the outcome for a given
patient. This situation has been described in several previous studies of depression predictors.7,18,79 Another
reason why clinicians are often unable to assess the usefulness of a predictor from research studies is the fact that
effect size estimates are not provided. One attempt to overcome this shortcoming has been the inclusion of odds ratios
or “number needed to treat” as part of the measurement of the outcome effect.
There have been attempts at evaluating predictors in terms of their usefulness to a practicing clinician.
Many of these attempts have also focused on changes occurring during the course of treatment (ie, process predictors).
Effective baseline predictors would be most useful for practicing clinicians since beginning patients on a treatment
that will work immediately is ideal. However, process predictors appear to have larger predictive power, thereby providing
clinicians with more relevant clinical utility.
CONCLUSION
Studies should report effect sizes in order to assist clinicians in
evaluating the strength of individual predictors. Furthermore, in order to evaluate the effectiveness of the predictor,
sensitivity as well as specificity of predictors should be reported so that both the positive and negative prediction
for a given variable can be quantified. Most studies of baseline predictors report the clinical significance of their
effects on treatment response, however this effect is modest. Thus, process predictors provide the most clinically
useful guide during treatment decision making. The utility of process predictors depends on the routine measurement
of variables using standardized rating instruments and has led to the development of measurement-based care as used
in the STAR*D study.
Disclosures: Dr. Trivedi is a consultant to AstraZeneca, Bristol-Myers Squibb, Cephalon, Cyberonics, Eli Lilly,
Forest, GlaxoSmithKline, Neuronetics, Novartis, VantagePoint, and Wyeth; is on the speaker’s bureaus of Bristol-Myers
Squibb, Cephalon, Cyberonics, Eli Lilly, Forest, GlaxoSmithKline, and Wyeth; and receives research support from Bristol-Myers
Squibb, Cephalon, Corcept, Cyberonics, Forest, the National Alliance for Research in Schizophrenia and Depression,
the National Institute of Mental Health, Novartis, Predix, and Wyeth.
Please direct all correspondence to: Madhukar H. Trivedi, MD, Mood Disorders Program and Clinic, Department of Psychiatry,
University of Texas Southwestern, 6363 Forest Park Rd, Suite 13.354, Dallas, TX 75390-9119; Tel: 214-648-0188; Fax:
214-648-0167; E-mail: [email protected].
References
1. Ustun TB. The global burden of mental disorders. Am J Public Health. 1999;89(9):1315-1318.
2. Stewart WF, Ricci JA, Chee E, Hahn SR, Morganstein D. Cost of lost
productive work time among US workers with depression. JAMA. 2003;289(23):3135-3144.
3. Kessler RC, Berglund P, Demler O, et al, and the National Comorbidity
Survey Replication. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication
(NCS-R). JAMA. 2003;289(23):3095-3105.
4. Olfson M, Marcus SC, Druss B, Elinson L, Tanielian T, Pincus HA.
National trends in the outpatient treatment of depression. JAMA. 2002;287(2):203-209.
5. Trivedi MH, Rush AJ, Wisniewski SR, et al, and the STAR*D Study
Team. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for
clinical practice. Am J Psychiatry. 2006;163(1):28-40.
6. Fava M, Davidson KG. Definition and epidemiology of treatment-resistant
depression. Psychiatr Clin North Am. 1996;19(2):179-200.
7. Rush AJ, Prien RF. From scientific knowledge to the clinical practice
of psychopharmacology: can the gap be bridged? Psychopharmacol Bull. 1995;31(1):7-20.
8. Trivedi MH, Morris DW, Pan JY, Grannemann
BD, Rush A. What moderator characteristics are associated with better prognosis for depression? Neuropsychiatr Dis
Treat. 2005;1(1):51-57.
9. Trivedi MH, Rush AJ, Pan JY, Carmody TJ. Which
depressed patients respond to nefazodone and when? J Clin Psychiatry. 2001;62(3):158-163.
10. Joyce PR, Mulder RT, Luty SE, et al. Patterns
and predictors of remission, response and recovery in major depression treated with fluoxetine or nortriptyline. Aust
N Z J Psychiatry. 2002;36(3):384-391.
11. Hirschfeld RM, Russell JM, Delgado PL, et al. Predictors of response
to acute treatment of chronic and double depression with sertraline or imipramine. J Clin Psychiatry. 1998;59(12):669-675.
12. Morishita S, Arita S. Possible predictors of response to fluvoxamine
for depression. Hum Psychopharmacol. 2003;18(3):197-200.
13. Katon W, Russo J, Frank E, et al. Predictors of nonresponse to treatment
in primary care patients with dysthymia. Gen Hosp Psychiatry. 2002;24(1):20-27.
14. Joyce PR, Mulder RT, Cloninger CR. Temperament predicts clomipramine
and desipramine response in major depression. J Affect Disord. 1994;30(1):35-46.
15. O’Leary D, Costello F, Gormley N, Webb M. Remission
onset and relapse in depression. An 18-month prospective study of course for 100 first admission patients. J Affect
Disord. 2000;57(1-3):159-171.
16. Kornstein SG, Schatzberg AF, Thase ME, et al. Gender differences
in treatment response to sertraline versus imipramine in chronic depression. Am J Psychiatry. 2000;157(9):1445-1452.
17. Bagby RM, Ryder AG, Cristi C. Psychosocial and clinical predictors
of response to pharmacotherapy for depression. J Psychiatry Neurosci. 2002;27(4):250-257.
18. Nierenberg AA. Predictors of response to antidepressants general
principles and clinical implications. Psychiatr Clin North Am. 2003;26(2):345-352.
19. Perlis RH, Alpert J, Nierenberg AA, et al. Clinical and sociodemographic
predictors of response to augmentation, or dose increase among depressed outpatients resistant to fluoxetine 20 mg/day. Acta
Psychiatr Scand. 2003;108(6):432-438.
20. Kocsis JH, Mason BJ, Frances AJ, Sweeney J, Mann JJ, Marin D. Prediction
of response of chronic depression to imipramine. J Affect Disord. 1989;17(3):255-260.
21. Joyce PR, Mulder RT, Luty SE, McKenzie JM, Rae AM. A differential
response to nortriptyline and fluoxetine in melancholic depression: the importance of age and gender. Acta Psychiatr
Scand. 2003;108(1):20-23.
22. Spijker J, de Graaf R, Bijl RV, Beekman AT, Ormel J, Nolen WA. Determinants
of persistence of major depressive episodes in the general population. Results from the Netherlands Mental Health Survey
and Incidence Study (NEMESIS). J Affect Disord. 2004;81(3):231-240.
23. Moses T, Leuchter AF, Cook I, Abrams M. Does the clinical course
of depression determine improvement in symptoms and quality of life? J Nerv Ment Dis. 2006;194(4):241-248.
24. Klein DN, Schatzberg AF, McCullough JP, et al. Age of onset in chronic
major depression: relation to demographic and clinical variables, family history, and treatment response. J Affect
Disord. 1999;55(2-3):149-157.
25. Diagnostic and Statistical Manual of Mental Disorders. 4th
ed, text rev. Washington, DC: American Psychiatric Association; 2000.
26. Brown WA. Treatment response in melancholia. Acta Psychiatr Scand
Suppl. 2007(433):125-129.
27. Peselow ED, Sanfilipo MP, Difiglia C, Fieve RR. Melancholic/endogenous
depression and response to somatic treatment and placebo. Am J Psychiatry. 1992;149(10):1324-1334.
28. Carroll BJ, Curtis GC, Mendels J. Neuroendocrine regulation in depression.
II. Discrimination of depressed from nondepressed patients. Arch Gen Psychiatry. 1976;33(9):1051-1058.
29. Ribeiro SC, Tandon R, Grunhaus L, Greden JF. The DST as a predictor
of outcome in depression: a meta-analysis. Am J Psychiatry. 1993;150(11):1618-1629.
30. Parker GB. Atypical depression: a valid subtype? J Clin Psychiatry.
2007;68(suppl 3):18-22.
31. Quitkin FM, Harrison W, Stewart JW, et al. Response to phenelzine
and imipramine in placebo nonresponders with atypical depression. A new application of the crossover design. Arch
Gen Psychiatry. 1991;48(4):319-323.
32. Liebowitz MR, Quitkin FM, Stewart JW, et al. Antidepressant specificity
in atypical depression. Arch Gen Psychiatry. 1988;45(2):129-137.
33. Quitkin FM, McGrath PJ, Stewart JW, et al. Atypical depression, panic
attacks, and response to imipramine and phenelzine. A replication. Arch Gen Psychiatry. 1990;47(10):935-941.
34. McGrath PJ, Stewart JW, Janal MN, Petkova E, Quitkin FM, Klein DF.
A placebo-controlled study of fluoxetine versus imipramine in the acute treatment of atypical depression. Am J Psychiatry.
2000;157(3):344-350.
35. Stewart JW, McGrath PJ, Quitkin FM. Do age of onset and course of
illness predict different treatment outcome among DSM IV depressive disorders with atypical features? Neuropsychopharmacology.
2002;26(2):237-245.
36. Fava M, Alpert JE, Carmin CN, et al. Clinical correlates and symptom patterns of anxious depression
among patients with major depressive disorder in STAR*D. Psychol Med. 2004;34(7):1299-1308.
37. Fava M, Rankin MA, Wright EC, et al. Anxiety disorders in major depression. Compr Psychiatry.
2000;41(2):97-102.
38. Fava M, Rush AJ, Alpert JE, et al. What clinical and symptom features and comorbid disorders
characterize outpatients with anxious major depressive disorder: a replication and extension. Can J Psychiatry.
2006;51(13):823-835.
39. Joffe RT, Bagby RM, Levitt A. Anxious and nonanxious depression. Am J Psychiatry. 1993;150(8):1257-1258.
40. Rush AJ, Zimmerman M, Wisniewski SR, et al. Comorbid psychiatric
disorders in depressed outpatients: demographic and clinical features. J Affect Disord. 2005;87(1):43-55.
41. VanValkenburg C, Akiskal HS, Puzantian V, Rosenthal T. Anxious depressions.
Clinical, family history, and naturalistic outcome--comparisons with panic and major depressive disorders. J Affect
Disord. 1984;6(1):67-82.
42. Cassano P, Soares CN, Cohen LS, Lyster AK, Fava M. Sex- and age-related
differences in major depressive disorder with comorbid anxiety treated with fluoxetine. Arch Womens Ment Health.
2004;7(3):167-171.
43. Fava M, Uebelacker LA, Alpert JE, Nierenberg AA, Pava JA, Rosenbaum
JF. Major depressive subtypes and treatment response. Biol Psychiatry. 1997;42(7):568-576.
44. Brown C, Schulberg HC, Madonia MJ, Shear MK, Houck PR. Treatment
outcomes for primary care patients with major depression and lifetime anxiety disorders. Am J Psychiatry. 1996;153(10):1293-1300.
45. Russell JM, Koran LM, Rush J, et al. Effect of concurrent anxiety
on response to sertraline and imipramine in patients with chronic depression. Depress Anxiety. 2001;13(1):18-27.
46. Perlis RH, Fraguas R, Fava M, et al. Prevalence and clinical correlates
of irritability in major depressive disorder: a preliminary report from the Sequenced Treatment Alternatives to Relieve
Depression study. J Clin Psychiatry. 2005;66(2):159-166.
47. Trivedi MH. The link between depression and physical symptoms. Prim
Care Companion J Clin Psychiatry. 2004;6(suppl 1):12-16.
48. Trivedi MH, Clayton AH, Frank E. Treating depression complicated
by comorbid medical illness or anxiety. J Clin Psychiatry. 2007;68(1):e01.
49. Iosifescu DV. Treating depression in the medically ill. Psychiatr
Clin North Am. 2007;30(1):77-90.
50. Perlis RH, Iosifescu DV, Alpert J, Nierenberg AA, Rosenbaum JF, Fava
M. Effect of medical comorbidity on response to fluoxetine augmentation or dose increase in outpatients with treatment-resistant
depression. Psychosomatics. 2004;45(3):224-229.
51. Patience DA, McGuire RJ, Scott AI, Freeman CP. The Edinburgh Primary
Care Depression Study: personality disorder and outcome. Br J Psychiatry. 1995;167(3):324-330.
52. Ezquiaga E, Garcia A, Bravo F, Pallares T. Factors associated with
outcome in major depression: a 6-month prospective study. Soc Psychiatry Psychiatr Epidemiol. 1998;33(11):552-557.
53. Ezquiaga E, Garcia-Lopez A, de Dios C, Leiva A, Bravo M, Montejo
J. Clinical and psychosocial factors associated with the outcome of unipolar major depression: a one year prospective
study. J Affect Disord. 2004;79(1-3):63-70.
54. Joyce PR, Paykel ES. Predictors of drug response in depression. Arch Gen Psychiatry. 1989;46(1):89-99.
55. Katon W, Lin E, von Korff M, et al. The predictors of persistence
of depression in primary care. J Affect Disord. 1994;31(2):81-90.
56. Papakostas GI, Iosifescu DV, Renshaw PF, et al. Brain MRI white matter
hyperintensities and one-carbon cycle metabolism in non-geriatric outpatients with major depressive disorder (Part
II). Psychiatry Res. 2005;140(3):301-307.
57. Kraft JB, Peters EJ, Slager SL, et al. Analysis of association between
the serotonin transporter and antidepressant response in a large clinical sample. Biol Psychiatry. 2007;61(6):734-742.
58. Malhotra AK, Murphy GM Jr, Kennedy JL. Pharmacogenetics of psychotropic
drug response. Am J Psychiatry. 2004;161(5):780-796.
59. Serretti A, Artioli P, Quartesan R. Pharmacogenetics in the treatment
of depression: pharmacodynamic studies. Pharmacogenet Genomics. 2005;15(2):61-67.
60. Bares M, Brunovsky M, Kopecek M, et al. Changes in QEEG prefrontal
cordance as a predictor of response to antidepressants in patients with treatment resistant depressive disorder: a
pilot study. J Psychiatr Res. 2007;41(3-4):319-325.
61. Cook IA, Leuchter AF, Morgan M, et al. Early changes in prefrontal
activity characterize clinical responders to antidepressants. Neuropsychopharmacology. 2002;27(1):120-131.
62. Cook IA, Leuchter AF, Witte E, et al. Neurophysiologic predictors
of treatment response to fluoxetine in major depression. Psychiatry Res. 1999;85(3):263-273.
63. Leuchter AF, Cook IA, Witte EA, Morgan M, Abrams M. Changes in brain
function of depressed subjects during treatment with placebo. Am J Psychiatry. 2002;159(1):122-129.
64. Perlis RH. Genetic predictors of antidepressant treatment response: progress towards clinical
pharmacogenetics. Primary
Psychiatry. 2007;14(6):53-58.
65. Rush AJ, Trivedi M, Fava M. Depression, IV: STAR*D treatment trial
for depression. Am J Psychiatry. 2003;160(2):237.
66. Trivedi MH, Baker SM. Clinical significance of monitoring early symptom
change to predict outcome. J Clin Psychiatry. 2001;62(suppl 4):27-33.
67. Nierenberg AA, McLean NE, Alpert JE, Worthington JJ, Rosenbaum JF,
Fava M. Early nonresponse to fluoxetine as a predictor of poor 8-week outcome. Am J Psychiatry. 1995;152(10):1500-1503.
68. Hamilton M. Development of a rating scale for primary depressive
illness. Br J Soc Clin Psychol. 1967;6(4):278-296.
69. Nierenberg AA, Farabaugh AH, Alpert JE, et al. Timing of onset of
antidepressant response with fluoxetine treatment. Am J Psychiatry. 2000;157(9):1423-1428.
70. Trivedi MH, Morris DW, Grannemann BD, Mahadi S. Symptom clusters
as predictors of late response to antidepressant treatment. J Clin Psychiatry. 2005;66(8):1064-1070.
71. Fava M, Rush AJ, Wisniewski SR, et al. A comparison of mirtazapine
and nortriptyline following two consecutive failed medication treatments for depressed outpatients: a STAR*D report. Am
J Psychiatry. 2006;163(7):1161-1172.
72. McGrath PJ, Stewart JW, Fava M, et al. Tranylcypromine versus venlafaxine
plus mirtazapine following three failed antidepressant medication trials for depression: a STAR*D report. Am J Psychiatry.
2006;163(9):1531-1541.
73. Nierenberg AA, Fava M, Trivedi MH, et al. A comparison of lithium
and T(3) augmentation following two failed medication treatments for depression: a STAR*D report. Am J Psychiatry.
2006;163(9):1519-1530.
74. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term
outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry.
2006;163(11):1905-1917.
75. Rush AJ, Trivedi MH, Wisniewski SR, et al. Bupropion-SR, sertraline,
or venlafaxine-XR after failure of SSRIs for depression. N Engl J Med. 2006;354(12):1231-1242.
76. Trivedi MH, Fava M, Wisniewski SR, et al, and the STAR*D Study Team.
Medication augmentation after the failure of SSRIs for depression. N Engl J Med. 2006;354(12):1243-1252.
77. Trivedi MH, Daly EJ. Measurement-based care for refractory depression:
A clinical decision support model for clinical research and practice. Drug Alcohol Depend. 2007;88(suppl 2):S61-S71.
78. Trivedi MH, Rush AJ, Gaynes BN, et al. Maximizing
the adequacy of medication treatment in controlled trials and clinical practice: STAR(*)D measurement-based care. Neuropsychopharmacology.
2007. In press.
79. Kraemer HC, Stice E, Kazdin A, Offord D, Kupfer D. How do risk factors
work together? Mediators, moderators, and independent, overlapping, and proxy risk factors. Am J Psychiatry.
2001;158(6):848-856.